Percutaneous Catheterization of the Internal Jugular Vein for Hemodialysis
Article information
Abstract
Objectives
The present study was aimed at evaluating the clinical experiences in the internal jugular venous catheterization for hemodialysis.
Methods
We retrospectively analyzed the data on internal jugular venous catheterization at Chonnam National University Hospital from May 2000 to Februrary 2001.
Results
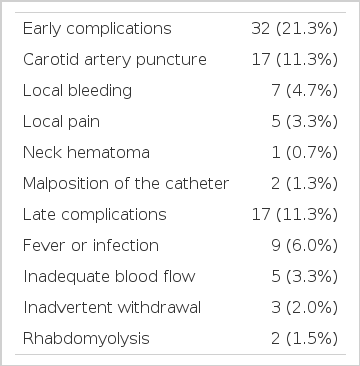
There were 132 uremic patients with a total of 150 attempts of internal jugular cannulation. Overall success rate was 90.9% with average puncture trials of 2.3±2.1. 124 (82.7%) of the catheterization attempts were made on the right side and 26 (17.3%) were made on the left. The catheters were left in place from 2 to 87 days with an average of 19.5±15.3 days per catheter. The dialysis sessions per catheter were from 2 to 58 with an average of 11.3±6.8. The mean blood flow during hemodialysis immediately after catheterization was 213.4±42.2 ml/min. Thirty two (21.3%) patients had early complications. These included carotid artery puncture (11.3%), local bleeding (4.7%), local pain (3.3%), neck hematoma (0.7%) and malposition of the catheter (1.3%). Seventeen (11.3%) patients had late complications. These included fever or infection (11.3%), inadequate blood flow rate (3.3%) and inadvertent withdrawal (2.0%). There was no catheter-related mortality.
Conclusions
Our experiences revealed that the internal jugular vein catheterization is relatively safe and efficient for temporary vascular access for hemodialysis.
INTRODUCTION
In patients with end-stage renal disease, cannulation of the central venous system with large-bore double-lumen catheters is often necessary until a functioning vascular access can be created. Despite recent technical advances in percutaneous venous cannulation, vascular access remains a major problem in patients requiring acute hemodialysis1).
Although a radiocephalic arteriovenous fistula appears to be the most satisfactory ‘permanent’ vascular access, it takes a long time before the fistula is available2). A temporary vascular access is, therefore, needed in patients with failure of a previous arteriovenous fistula or in patients requiring prompt hemodialysis due to acute renal failure. A dual-lumen internal jugular vein dialysis catheter is now widely used for this purpose because of both reliability and safety3). However, the technique of placing a dual-lumen catheter is not without complication.
Herein we report our experiences with internal jugular vein catheterization for hemodialysis with a review of the literature.
MATERIALS AND METHODS
We retrospectively analyzed the data on internal jugular dialysis catheter insertion at Chonnam University Hospital from May 2000 through February 2001. There were 132 patients with a total of 150 attempts of internal jugular cannulation, and successful cannulations were obtained in 135 lines. All lines were placed by either trainee nephrology fellows or residents, under attending supervision.
Landmark cannulation of the internal jugular vein was performed after the skin at the top of the triangle between the sternal and clavicular head of the sternocleidomastoid muscle was anesthesized with 1% lidocaine. A 21-gauge finder needle connected to a 10-mL syringe was advanced through the skin at an approximately 60° angle and in the direction of the right nipple. After aspiration of venous blood, the finder needle was used to guide an introducer cannula connected to a 10-mL syringe. On recannulation of the vein, a guidewire was advanced into the vein. The needle was removed and a noncuffed dual-lumen catheter was advanced over the wire into the internal jugular vein. A chest X-ray was performed at the end of the procedure and reviewed by a radiologist to confirm that no complications had occurred.
RESULTS
From May 2000 to February 2001, there were 132 uremic patients with a total of 150 attempts of internal jugular cannulation and successful cannulations were obtained in 135 lines. The age of the patients was from 16.5 to 75.0 years old with an average age of 47.2. Seventy one patients (53.8%) were men, and sixty one (46.2%) were women. 124 (82.7%) of the catheterization attempts were made on the right side and 26 (17.3%) were made on the left. Overall success rate was 90.9% with average puncture trials of 2.3±2.1. The success rate at the first, second and third or more attempts was 52.7%, 42% and 19.3%, respectively. The catheters were left in place from 2 to 87 days with an average of 19.5±15.3 days per catheter. The dialysis sessions per catheter were from 2 to 58 with an average of 11.3±6.8. The mean blood flow during hemodialysis immediately after catheterization was 213.4±42.2 mL/min (Table 1).
Among the 132 patients, the underlying renal diseases include diabetic nephropathy in 42 (31.8%), chronic glomerulonephritis in 38 (28.8%), hypertensive nephrosclerosis in 19 (14.4%), obstructive nephropathy in 9 (6.8%), lupus nephritis, in 4 (3.0%), polycystic kidney disease in 2 (1.5%), rapidly progressive glomerulonephritis in 1 (0.8%), postoperative acute renal failure (ARF) in 7 (5.3%), ARF due to multiorgan failure in 5 (3.8%), ARF due to snake bite in 2 (1.5%) and ARF due to rhabdomyolysis in 2 cases (1.5%) (Table 2).
Thirty two (21.3%) patients had early complications. These included carotid artery puncture in 17 (11.3%), local bleeding in 7 (4.7%), local pain in 5 (3.3%), neck hematoma in 1 (0.7%) and malposition of the catheter in 2 (1.3%). Seventeen (11.3%) patients had late complications. These included fever or infection in 9 (6.0%), inadequate blood flow rate in 5 (3.3%) and inadvertent withdrawal in 3 cases (2.0%) (Table 3).
The causes of removal of catheters were as follows; no more need due to AV fistula in 85 (56.7%), continuous ambulatory peritoneal dialysis in 45 (30.0%), recovery from acute renal failure in 10 (6.7%), exit site infection in 3 (2.0%), neck hematoma in 1 (0.7%), exit site bleeding in 1 (0.7%), accidental slip out in 2 (1.3%) and hopeless discharge due to multiorgan failure in 3 cases (2.0%) (Table 4).
DISCUSSION
Double-lumen catheters are widely used for temporary access to the circulation in patients who require acute hemodialysis (HD). Since HD requires rapid extracorporeal blood flow, femoral, subclavian and internal jugular veins are the sites most commonly chosen4).
Several factors preclude the wider use of indwelling femoral HD catheter, including interference with ambulation and concern over bleeding, infection and deep venous thrombosis5).
The subclavian vein (SCV) route emerged as the most commonly used site. However, reports of complications, both short- and long-term, associated with SCV catheters raised concern6–8). Furthermore, the occurrence of chronic SCV stenosis with its adverse consequences on the ipsilateral permanent access is particularly worrisome. Cimochowski et al.3) suggested that, compared to the internal jugular vein (IJV) route, the long-term stricture rate of SCV catheters was unacceptably high.
Internal jugular vein cannulation has become the preferred approach for temporary hemodialysis catheter placement following the reports of an increased incidence of subclavian vein stenosis due to subclavian vein catheterization9, 10).
Success rates and incidences of traumatic complications associated with the internal jugular venous cannulation have been reported elsewhere. In the study performed by Jobes et al.11) a success rate of 93.2% and an arteriotomy incidence of 3.9% were reported. In a series of 302 patients, Denys et al.12 discovered a success rate of 88.1% and a carotid puncture rate of 8.3%. Vanholder et al.13) reported an incidence of 3.7% for traumatic complications, and Campistol et al.14) discovered an arteriotomy rate of 4%.
The average number of needle passes and the success rate of the first punctural attempt are of concern because an increasing number of needle passes contributed to a higher incidence of traumatic complications12). In our experience, the average number of puncture trials was 2.3±2.1 times. The success rate was 82.7% and the carotid arterial puncture rate was 11.3%, similar to the published data. Among our patients, local hematoma with neck swelling developed in a patient after 24 hours of catheter insertion. The patient received multiple puncture trials (5 attempts) without arterial puncture. The blood flow was adequate for hemodialysis initially. There was no symptom or sign of airway narrowing or obstruction. The neck CT showed large hematomas in the prevertebral space, bilateral carotid spaces and deep cervical fascial pianes (Figure. 1) and circumferential hematomas surrounding the trachea and major supra-aortic vessels (Figure. 2) and the catheter adhering to the right internal jugular vein (arrow). Although the catheter tip was not noticed in the CT scan, we surmise that the laceration of the vessel wall may be related to the formation of neck hematoma. We removed the catheter at a moment’s notice of neck swelling, and the hematoma resolved spontaneously during 48 hours with close observation.
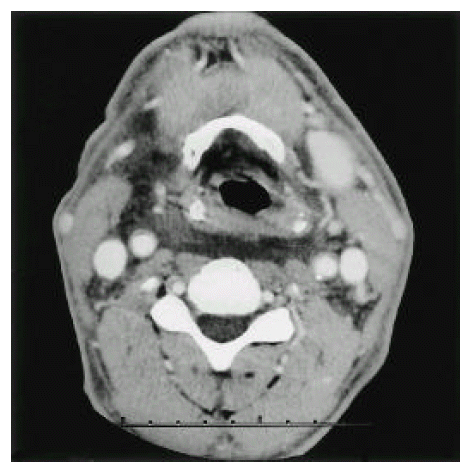
Neck CT shows large hematomas in prevertebral space, bilateral carotid spaces and deep cervical fascial planes
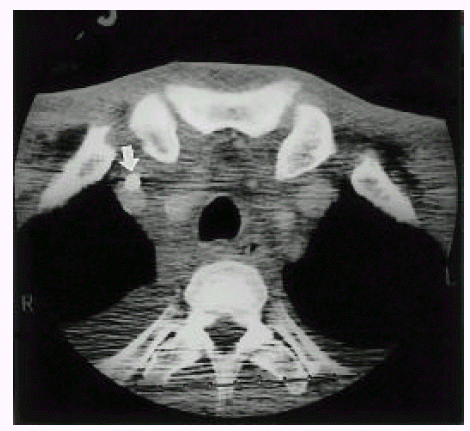
Neck CT shows circumferential hematoma surrounding trachea and major supra-aortic vessels. Note the catheter adhering to the right internal jugular vein (arrow).
For uremic patients, the incidence of unilateral anatomical variations of the internal jugular veins has been reported to be 17.3% and that of bilateral anatomical variations 8.7%15). This might explain why a high rate of traumatic complications occurred in some uremic patients who underwent external landmark-guided internal juguiar venous cannulation, even though the cannulating procedure was performed by an experienced operator. Recently, the ultrasound-guided technique has been utilized to improve the success rate and to reduce the complication rate of central venous catheterization in medical and surgical patients. Lin et al.16) evaluated the value of ultrasound technique and demonstrated the superiority of the ultrasound technique as compared with the landmark-guided technique in creating temporary dialysis hemoaccess.
The overall incidence of complications in IJV catheterization is 0.1–4.2%, with a few studies reporting higher incidences17). Significant complications include internal carotid artery (ICA) puncture pneumothorax, vessel erosion, thrombosis, infection and even life-threatening airway obstruction17, 18). By far the most common complication is ICA puncture17). Vertebral artery pseudoaneurysm1, 19), serious fatal vascular injuries involving the vertebral artery and ascending cervical artery leading to hemothorax and exsanguination have also been reported20, 21).
In our patients, early complications, such as carotid artery puncture (11.3%), local bleeding (4.7%), local pain (3.3%), neck hematoma (0.7%) and malposition of the catheter (1.3%) developed in 21.3%. Late complications, such as infection (6.0%), inadequate blood flow (3.3%) and inadvertent withdrawal (2.0%) developed in 11.3% of our patients. The rate of infection for internal jugular or subclavian noncuffed catheters suggests that they should be used for no more than 3 weeks22). In our study, the mean duration of catheter placement 19.5±15.3 days per catheter. Ipsilateral arteriovenous accesses were created in 75 patients with formerly internal jugular vein catheterization.
Previously, we had reported the complications in subclavian vein catheterization for hemodialysis8), including pneumothorax (1.7%), hemothorax (1.0%), hemopneumothorax (0.4%), subclavian vein thrombosis (0.7%) and subclavian vein stenosis (1.7%). In the present study, however, none of them had increased venous dialysis pressures or persistent arm swelling after shunt surgery. Neither pneumothorax nor hemothorax was detected. There was no catheter related mortality.
In conclusion, our experiences revealed that the percutaneous internal jugular vein catheterization with a double lumen catheter is a relatively safe and efficient temporary vascular access for hemodialysis.