Drug-resistant tuberculosis in a tertiary referral teaching hospital of Korea
Article information
Abstract
Background :
Resistance of Mycobacterium tuberculosis strains is an increasing problem worldwide. Our purpose was to determine the prevalence of drug resistance (DR) and risk factors of DR in patients with tuberculosis and to assess the clinical characteristics and socioeconomic status of patients with drug-resistant tuberculosis.
Methods :
We retrospectively studied drug susceptibility tests and clinical and socioeconomic records for 308 cases of culture-positive Mycobacterium tuberculosis infection, diagnosed at Mokdong Hospital, Ewha Womans University from March, 1995 to February, 2000.
Results :
DR to at least one drug was identified in 75 (24.4%); the rate of primary DR, 18.7% and acquired DR, 39.3%. Multi-drug resistance (MDR) was identified in 31 (10.1%); primary MDR, 7.0% and acquired MDR, 21.4%. The risk factors of DR were previous TB treatment, pulmonary involvement and associated medical illness. DR group showed lesser adherence to treatment than the drug-sensitive group. DR group showed more frequent self-interruption of medication, lower completion rate of treatment and higher failure rate of follow-up than the drug-sensitive group. In previously treated tuberculosis patients, higher rate of overall DR and MDR, larger number of resistant drugs and more frequent self-interruption of medication were observed than newly diagnosed patients. Among DR group, acquired DR (ADR) group was older, less educated and treated for longer duration and had more advanced disease than primary DR group.
Conclusion :
Previously treated tuberculosis is a most important risk factor for DR. DR group, especially ADR, showed less compliance with treatment. More proper education and attention to prevent self-interruption should be given to a previously treated group. In TB prevalent areas, it should be considered to obtain initial drug susceptibility testing in high risk of DR.
INTRODUCTION
Tuberculosis (TB) is one of the major infectious diseases in severity and morbidity despite coverage of the National Tuberculosis Program and improved social and economic conditions1). Recently, epidemic of acquired immune deficiency syndromes, poor isolation of drug-resistant subjects and increased numbers of poor and crowded residences have caused increased proportion of drug resistance (DR), especially multi-drug resistance (MDR)2–7). In Korea, although the overall prevalence of tuberculosis diminished to less than 1.0%, the rate of drug resistance, especially MDR in previously treated groups was still high8, 9). It is important to define the risk factors, including the socioeconomic status of DR for the prompt isolation and proper management of drug-resistant tuberculosis. We examined current characteristics of DR in a university hospital setting and compared it to national studies.
MATERIALS AND METHODS
1. Study population
From March 1995 through February 2000, 308 patients with culture-positive Mycobacterium tuberculosis infection and their drug susceptibility testing were included. The medical records of these patients were reviewed and the following data were collected: age, sex, history of previous antituberculous treatment, familial history of TB, associated medical illness of diabetes mellitus, liver disease and malignancy, history of smoking and alcohol drinking, marital status, and employment status. Whether they graduated more than high school and had resided in their own houses were inquired of patients with drug resistant tuberculosis using interviews by letters or telephones.
2. Drug susceptibility test
The drug susceptibility testing against ten antituberculosis drugs was conducted by a laboratory certified under the Korean National Tuberculosis Association. The drug susceptibility of the M. tuberculosis isolates was determined by the absolute concentration method described in detail by Canetti et al10). The drugs and their critical concentrations for resistance are as follows: isoniazid 0.2 μg/mL; rifampin 40 μg/mL; ethambutol 2 μg/mL; streptomycin 10 μgg/mL; kanamycin 40 μg/mL; prothionamide 20 μg/mL; cycloserine 30 μg/mL; paraam-inosalicylic acid 1 μg/mL; ofloxacin 2 μg/mL. Pyrazinamide susceptibility was determined by pyrazinamidase test.
3. Definitions
The term ‘previously treated TB patients’ refers to patients with verified TB with treatment of more than one month in the past. The term ‘drug resistance’ means resistance to at least one antituberculosis drug, ‘poly-drug resistance’ means resistance to more than any two drugs and ‘multi-drug resistance’ means resistance to at least isoniazid and rifampin. The term ‘primary DR’ refers to resistance occurred in a patient who has never received antituberculosis therapy and ‘acquired DR’ refers to resistance developed during or following chemotherapy of subjects who had previously been regarded as drug-susceptible tuberculosis.
4. Data analysis
The data were analyzed using SPSS software. The χ2 test and independent T test were applied to compare variables between the two groups. Multiple logistic regression analysis was used to define the strength of major risk factors to develop DR.
RESULTS
1. Rate of drug resistance
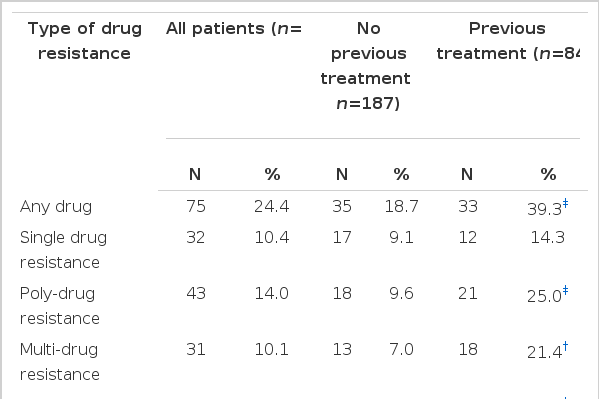
Among 308 culture-positive patients, 187 had newly diagnosed TB and 84 had previously treated TB, except for 37 cases with no available record of previous TB history. Resistance to at least one drug was found in 75 (24.4%) out of 308 cases and MDR in 31 (10.1%). Among antituberculosis drugs, resistance to isoniazid was the most common (19.5%), followed by resistance to rifampin (12.3%), ethambutol (8.8%) and streptomycin (5.8%). Primary DR was found in 35 (18.7%) out of 187 cases and primary MDR in 13 (7.0%). Primary DR to isoniazid was 13.4%; rifampin, 9.1%; ethambutol, 7.0% and streptomycin, 4.8%. Acquired DR was found in 33 (39.3%) out of 84 cases and acquired MDR in 18 (21.4%). Acquired DR to isoniazid was 35.7%; rifampin, 22.6%; ethambutol, 15.5% and streptomycin, 8.3%. Each of acquired DR and acquired MDR was significantly higher than each of primary DR and primary MDR (39.3% vs. 18.7%; p<0.001, 21.4% vs. 7.0%; p<0.01) (Table 1).
2. Comparison between drug-sensitive group and drug-resistant group
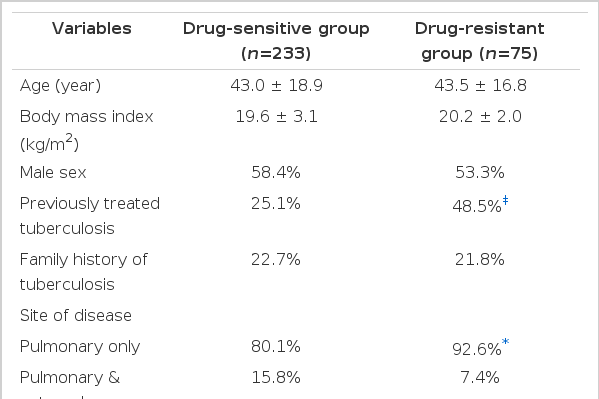
Drug-resistant group presented a history of previously treated TB (48.5% vs. 25.1%; p<0.001), pulmonary involvement of TB (92.6% vs. 80.1%; p<0.05) and associated medical illness more frequently than drug-sensitive group (29.4% vs. 19.5%; p<0.05). Drug-resistant group showed more frequent self-interruption of medication (38.8% vs. 13.4%; p<0.001), lower completion rate of treatment (39.7% vs. 58.4%; p<0.01) and higher failure rate of follow-up than drug-sensitive group (36.8% vs. 12.7%; p<0.001) (Table 2).
3. Risk factors of drug resistance
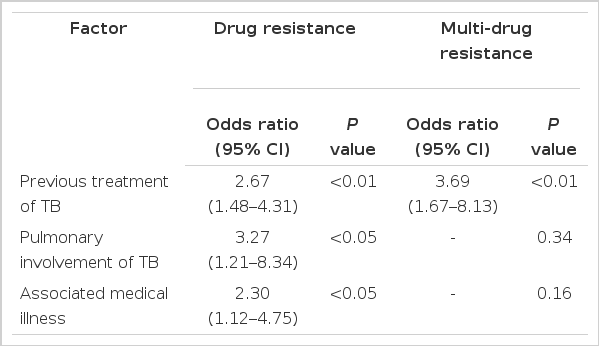
Multiple logistic regression analysis was applied to factors associated with DR and MDR. Previous treatment of TB was the strongest risk factor of DR (odds ratio, 2.67; p<0.01) and MDR (odds ratio, 3.69; p<0.01). Pulmonary involvement of TB (odds ratio, 3.27; p<0.05) and associated medical illness (odds ratio, 2.30; p<0.05) were also risk factors of DR, but not of MDR (Table 3).
4. Comparison between primary DR and acquired DR
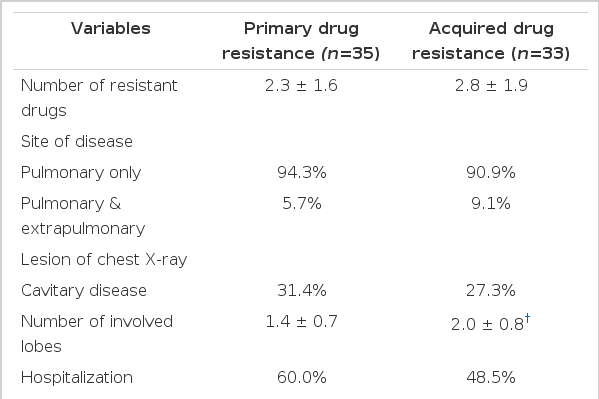
Acquired DR group was older (48.2 ± 16.5years vs. 39.6 ± 16.3years; p<0.05) and lesser educated than primary DR group (11.1% vs. 38.9%; p<0.05). Acquired DR group also had higher rate of family history of TB (28.0% vs. 16.7%) and more lived in rented houses than primary DR group (30.8% vs. 22.2%), but it is not statistically significant (Table 4). Acquired DR group showed more involved lobes in chest X-ray (2.0 ± 0.8 vs. 1.4 ± 0.7; p<0.01) and longer duration of treatment than PDR group (18.3 ± 7.2 months vs. 10.6 ± 6.3 months; p<0.05) (Table 5).
DISCUSSION
This study presented findings related to drug resistance in a TB endemic country not associated with HIV epidemic. The drug resistance in TB is one of the most important problems in the world and a major cause of morbidity and mortality2). TB was one of the most common 10 causes of Korean adults’ deaths until 19968). DR and severity of infection are important factors determining the mortality in TB patients. The national goal of TB control is closely related to control of DR. According to a national TB survey of Korea, the rate of DR was the highest in 1980 and then it tended to decline8, 9).
The rate of isoniazid-resistant TB was the highest among the antituberculous drugs throughout the world and the rate of rifampin-resistant TB was high in an endemic area5,11–14). This study showed that nearly 20 percent of the patients with active TB could transmit isoniazid-resistant organisms. The use of rifampin with or without pyrazinamide in chemoprophylaxis for the case contact in a prevalent area of isoniazid-resistant TB is issued15). The increasing resistance to rifampin is particularly troubling, since rifampin is essential to short-course antituberculosis therapy16). In this study, 10 percent of the total cases could transmit organisms resistant to both isoniazid and rifampin. Consensus on preventive therapy for MDR TB was not reached and the current recommendation was a regimen of pyrazinamide and fluoroquinolone for 4 months17).
Because previous use of antituberculous medication was the most important risk factor of DR4,6,13,14,18,19) as shown in this study, and accompanied medical illness due to decreased immunity was high risk group of DR3, 20, 21), DR should be suspected in cases of delayed response to therapy or continued positive AFB smear despite treatment. The ability to complete antituberculosis therapy can be affected by housing status, employment status, level of literacy and psychological aspects22–25). In this study, alcohol abuse, marital status or employment status did not influence the development of DR. Because patients with DR are usually treated with 2nd line antituberculosis drugs for a long duration, which are more toxic and costlier than first line drugs, they are apt to have poor adherence to medication compared with drug-sensitive patients. The other factors to develop DR are drug intolerance during therapy25), contact with DR TB26), birth and residence in an endemic area5,6,13,14,19), HIV infection6, 13) and cavitary pulmonary tuberculosis18, 19).
The first large scale sample survey in Korea in 1994, cooperation with WHO and IUATLD9, 11), presented lower rates in overall DR and primary DR and higher rate in acquired DR compared with this study (Table 6). The discrepancies between this study and the nationwide study could originate from differences in the size and nature of the study population, study period and study area. The population of previous treated TB cases out of all cases in this study was higher than that in the national study (84 of 308 cases, 27.3% vs. 189 of 2675 cases, 7.1%). This could contribute to raise the overall rate of DR in this study. Although the rate of acquired DR was lower in this study than the national survey of 1994, the rate of MDR is about 3 times that of the national survey of 1994. The population of this study was derived mainly from residents in Seoul. Residents in other areas were only 52 (13.6%) out of 308 patients. Therefore, this study could represent drug resistance in Seoul as an urban area. Generally, an urban community is more crowded and has more chance to transmit an infectious disease than a rural community. As another reason to be considered, more advanced cases among newly diagnosed subjects might prefer to be treated in 3rd referral hospitals rather than in a public TB care center as in rural areas. Actually, in this study, patients with primary DR showed a higher rate of hospitalization despite a younger age than those with acquired DR, and the rate of primary MDR in this study was far higher than that in the national survey of 1994. Our data had comparable results with the report from Chungnam University of Korea27).
In this study, patients with acquired DR showed chronic reluctant course, low education level and low economic status compared with those with primary DR. Traditionally, patients with DR TB are classified as having acquired DR or primary DR on the basis of a history of previous TB treatment28, 29). Only cases of primary DR are assumed to be due to transmission of DR strains. Recent studies based on restriction-fragment length polymorphisms, useful in distinguishing different strains of M. tuberculosis, revealed that recent transmission as well as true acquisition of DR during therapy was an important cause of DR, especially MDR, even in previously treated cases in certain urban populations30, 31). Therefore, traditional clinical classification based on a history of previous treatment may result in misinterpretation and the underestimation of transmission. Improper isolation and poor ventilation due to crowding are important reasons for spread of TB particularly in the low socioeconomic status of urban areas. The resistant cases, especially previously treated, remain sources of infection for prolonged periods and they are likely to infect others31–35). Finally, the high rate of DR and MDR in acquired cases contribute to increasing rates of DR and MDR in primary cases.
When dealing with a patient who has resistant TB, it is very important that the physician evaluate the situation fully. When seeing a patient who has already been treated for TB, susceptibility tests should be ordered. The only way to ensure that patients take their medication properly is to give it to them, i.e. direct observed therapy (DOT)34). Although routine DOT can not be afforded in many countries with a high prevalence of MDR TB, application of DOT is requisite in drug resistant TB, especially for poor adherence to treatment, to prevent the development of further drug resistance36).
Previously treated tuberculosis is a most important risk factor for DR. DR group, especially ADR, showed less compliance with treatment. More proper education and attention to prevent self-interruption should be given to a previously treated group. This study showed a higher rate of primary DR and primary MDR compared to the national study, suggesting more severe cases among newly diagnosed patients treated in tertiary referral hospitals rather than public TB care centers. High rate of acquired DR contributes to increasing the rate of primary DR. Initial drug susceptibility testing is necessary to guide optimal treatment to a culture positive case with the high risk of DR, especially in TB prevalent areas.