Kidney Transplantation in Patients with Type 1 Diabetes Mellitus: Long-Term Prognosis for Patients and Grafts
Article information
Abstract
Kidney transplantation is the best therapeutic choice to improve survival and quality of life in patients with end-stage diabetic nephropathy. Long-term prognosis in diabetic patients who received kidney transplants, however, has not been delineated. We, therefore, studied patient and graft survival, graft function and cause of graft failure in 78 Type I diabetic kidney transplant recipients in The Rogosin Institute/The Weill-Cornell Medical Center, New York who had functioning grafts for more than one year. The results were compared with 78 non-diabetic patients who had functioning grafts for more than one year and were matched for age, gender, donor source, time of transplantation and immunosuppressive therapy protocol. Cumulative patient survival rates for diabetic patients were significantly lower than those of non-diabetic patients (86% vs. 97% at 5 years and 74% vs. 95% at 10 years, respectively: p<0.05). The most common cause of death was cardiovascular disease. Graft survival rates for diabetic patients were also lower than that of non-diabetic patients (71% vs. 80% at 5 years and 58% vs. 72% at 10 years, respectively), but the differences did not reach statistical significance. Among the 22 failed grafts in diabetic patients, 7 (32%) were due to patient death rather than primary graft failure. If the patients who died with a functioning graft were censored, graft survival rates of diabetic patients approached those of non-diabetic patients (80% vs. 81% at 5 years and 65% vs. 73% at 10 years, respectively). Creatinine clearances in diabetic patients were lower than that in non-diabetic patients through the follow-up period, but the differences were significant only for the first few years. At no time was there a higher creatinine clearance for diabetic patients. Among the 16 patients who had transplant kidney biopsies two to seven years post-transplant, 6 showed morphological changes consistent with diabetic nephropathy. One patient lost graft function solely by recurrent diabetic nephropathy. We conclude that long-term patient survival for diabetic patients is significantly lower than that of non-diabetic patients, due primarily to cardiovascular disease. Graft survival is comparable between the two groups. Creatinine clearances of diabetic patients are lower than those of non-diabetic patients. There is no apparent glomerular hyperfiltration at any time in diabetic patients. Recurrence of diabetic nephropathy is a rare cause of graft failure in the first 10 year post-transplant period. Aggressive intervention to modify cardiovascular risk factors should improve patient and graft survival in diabetic kidney transplant recipients.
INTRODUCTION
Diabetes mellitus is the most common cause of end-stage renal disease (ESRD) in the U.S.A. and most industrialized countries1). Type I diabetic patients with end-stage diabetic nephropathy requiring dialysis have a poor prognosis due to continued progression of neuropathy, enteropathy, retinopathy and peripheral vascular disease2). Progression of these complications compromises quality of life and limits survival1, 3). Kidney transplantation, on the other hand, provides these patients with a better survival rate4,5), as well as a greatly improved quality of life6–9). In a review of 100 diabetic kidney transplant recipients who survived more than 10 years with a functioning allograft, Najarian et al stated10): “For diabetic patients, renal function is essential for survival, unlike the non-diabetics who can survive on dialysis. Thus renal transplantation is the treatment of choice for diabetic nephropathy”. There are, however, limited studies on the long-term prognosis of patient and graft survival and the risk of recurrence of diabetic nephropathy11, 12). Previously, we reported that patient and graft survival rates of Type I diabetic kidney transplant recipients were comparable to those of non-diabetic patients for the first 5 years6). We also reported that these patients had a marked improvement in physical performance and quality of life after successful kidney transplantation. In the present study, we evaluated long-term patient and graft survival, graft function and the recurrence rate of diabetic nephropathy in Type I diabetic kidney transplant recipients. The results were compared with matched controls of non-diabetic kidney transplant recipients.
MATERIALS AND METHODS
1. Study Subjects
Study subjects include 78 patients with Type I diabetes mellitus and end-stage diabetic nephropathy who underwent a first kidney transplant between 1980 and 1996 in The Rogosin Institute/The Weill-Cornell Medical Center, New York, and had a functioning renal allograft for more than one year. An equal number of non-diabetic patients with functioning renal allografts for more than a year, matched for recipient age, gender, donor source (living-related or cadaveric donor), time of transplantation and immunosuppressive therapy protocol, were selected as controls. Since this study focused on the long-term prognosis of diabetic kidney transplant recipients, the subjects were limited to those who had functioning allografts for more than one year. By doing so, the impact of risk factors other than diabetes, such as delayed graft function, acute transplant rejection and drug nephrotoxicity could be minimized. Most patients were treated with triple immunosuppressive therapy consisting of prednisone, azathioprine and cyclosporine, without antilymphocyte antibody induction therapy. Details of the immunosuppressive protocol were described previously13). Seventy five to eighty percent of patients in both groups were treated with antihypertensive drugs, most commonly with long-acting nifedipine, not only to control blood pressure, but also to minimize the adverse renal effects of cyclosporine13). A small number of patients in both groups received an angiotensin converting enzyme inhibitor for hypertension during the study period. The pre-transplant cardiac evaluation included medical review for symptoms and signs of cardiovascular disease, resting EKG, echocardiogram and stress tests, if indicated.
2. Methods
Survival rates were calculated over the ensuing 9 years assuming the first year post-transplant as the starting point. In analysis of graft survival rates, both loss of graft function and death with a functioning graft were considered as graft failure. Additional graft survival rates were also calculated, censoring patients who died with a functioning allograft. In the patient survival analysis, all deaths, including those who died after loss of graft function and who had returned to dialysis, were included in the analysis. Creatinine clearances were estimated by the Cockcroft-Gault formula14). This formula for the estimation of creatinine clearance has been validated in both diabetic patients with nephropathy15) and diabetic kidney transplant recipients16). The Student’s t-test, chi-square test, Kaplan-Meier method and Log-Rank test, where applicable, were used for statistical analysis. p equal to or less than 0.05 was considered to be significant.
RESULTS
Demographic data of the recipients, kidney donors, incidences of delayed graft function (for cadaveric kidney recipients only) and duration of follow-up for the study subjects are shown in Table 1. Since non-diabetic patients were chosen to match diabetic patients, the mean age, gender and donor source (living-related/cadaveric donor) of diabetic patients were comparable to those of non-diabetic patients. The incidence of delayed graft function in cadaveric kidney recipients and rejection episodes in the first year were comparable between the two groups.
1. Patient Survival Rates
Patient survival rates for diabetic patients were 86% and 74% at 5 and 10 years post-transplant, respectively. Non-diabetic patient survival rates were 97% and 95%, respectively (Figure 1). The differences in the patient survival rate were statistically significant (p<0.05). Complications of cardiovascular disease (58%) were the most common cause of death in diabetic patients. Other causes included sepsis (8%), withdrawal from dialysis treatment after graft failure (8%) and other miscellaneous causes (Table 2).
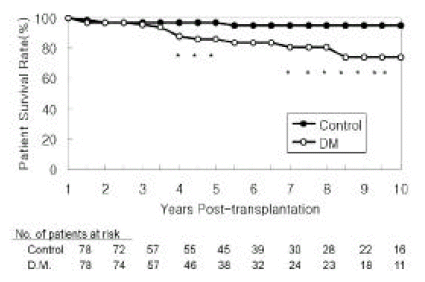
Patient Survival Rates
Survival rates were calculated over the ensuing 9 years assuming the first year post-transplant as the starting point. Asterisk indicates the difference in survival rates between the two groups is statistically significant (p<0.05).
2. GRAFT SURVIVAL RATES
Graft survival rates for diabetic patients were 71% and 58% at 5 and 10 years post-transplant, respectively, and 80% and 72%, for non-diabetic patients (Figure 2). Although non-diabetic patients tend to have better graft survival rates, the differences were not statistically significant. In diabetic patients, chronic rejection was the most common cause of graft failure. Surprisingly, 32% of graft failures were a result of the patient’s death rather than primary graft failure. Only one patient (5%) lost graft function primarily due to the recurrence of diabetic nephropathy. When patient deaths with a functioning graft are censored, graft survival rates of diabetic patients (80% and 65% at 5 and 10 years, respectively) approach that of non-diabetic patients (81% and 73%, respectively). Sixteen patients underwent renal graft biopsy at 2 to 7 years after transplantation (4.4 +/− 3.3 years) for unexplained azotemia and/or proteinuria. In these, six grafts (37.5%) showed pathological changes (mesangial expansion, glomerular sclerosis, basement membrane thickening) consistent with diabetic nephropathy. However, only one of them lost graft function two years after biopsy as a result of recurrent diabetic nephropathy (8 years post-transplant).
3. Graft Function
Creatinine clearance was stable, albeit lower in diabetic patients than non-diabetic patients throughout the 10 year observation period (Figure 3). The differences between the two groups were statistically significant at 2 years (p<0.02), 2.5 years (p<0.004) and 3 years (p<0.01). At no time did the diabetic patients have higher creatinine clearances than the non-diabetic patients.

Creatinine Clearance
Creatinine Clearance of the two groups of patients. Asterisk indicates the differences of creatinine clearances between the two groups are statistically significant (*p<0.05, **p<0.01).
Thus, there was no evidence of hyperfiltration in this group of diabetic kidney transplant recipients.
DISCUSSION
Diabetic patients with ESRD present a difficult challenge for medical and surgical treatment. A successful kidney transplant for Type I diabetic patients with ESRD appears to provide better survival4, 5) and improved rehabilitation than continued dialysis treatment2, 6, 7). Kidney transplantation is, therefore, the treatment of choice for both Type I and Type II diabetic patients with ESRD9). In 1996, 24% of 12,000 patients who received kidney transplantation in the United States were diabetic1). Diabetic nephropathy is not only the most common cause of ESRD requiring dialysis treatment but it is also the most common kidney disease leading to kidney transplantation. This study examines how patients with Type I diabetes fare after kidney transplantation in terms of patient survival, graft survival and graft function on a long-term basis, as compared with non-diabetic patients.
We found that both short-term (5 years), and long-term survival (10 years) of diabetic patients was significantly worse than that of non-diabetic patients (74% vs. 95%, respectively, at 10 years). A similar result was reported by Kumar et al.11), who reviewed 52 Type I diabetic transplant patients. The actuarial patient survival rate at 5 years was significantly lower in diabetic patients than in non-diabetics. The difference in survival rates was primarily caused by a higher incidence of cardiovascular deaths. Perez et al., who reviewed 3,000 kidney transplants at the University of Minnesota17, 18) also noted a significantly lower patient survival for diabetic patients five years post-transplant. In addition, they found that cardiovascular disease was the most common cause of death in diabetic patients10, 11, 17, 18). Hirsch et al. also observed myocardial infarction and sepsis were the main cause of death in Type II diabetes mellitus transplant recipients9). Significant coronary artery disease is found in one-third to one-half of ESRD diabetic patients undergoing evaluation for transplantation19). Since coronary artery disease is so prevalent among diabetic patients with renal failure19–21), a preoperative cardiovascular evaluation should be carried out even for asymptomatic patients, both to identify high risk patients and to improve post-transplant patient survival19, 22–24). Aggressive pre-transplant cardiac assessment and cardiovascular intervention can improve post-transplant morbidity and mortality in diabetic patients11, 12, 25).
Long-term graft survival in diabetic patients was also lower than that in non-diabetic patients (58% vs. 72%, respectively, at 10 years), but the difference was not statistically significant. A similar result has been observed by the Minnesota group17) since the introduction of cyclosporine in the early 1980’s (71% vs. 70%, respectively, at 5 years). Ekberg et al found, in a review of 189 uremic diabetics, that the overall patient survival rate was similar between diabetic and non-diabetic groups transplanted after 198812). In this and other studies17, 26), many patients died with functioning grafts. Death was, therefore, the major cause of graft failure. If these patients were censored, graft survival rates were comparable (65% for diabetic patients vs. 73% for non-diabetic patients at 10 years). It is interesting to note that diabetes per se appears to impose minimal adverse effects on graft survival, at least for the first decade. Aggressive preventive intervention in diabetic patients before and after transplantation for cardiovascular risk factors should help improve both patient and graft survival.
Changes in glomerular filtration rates in Type I diabetic patients and the pathophysiology of diabetic nephropathy have been extensively studied. Few studies, however, have examined the functional changes of renal allografts transplanted in diabetic patients16, 27). Over the 10 years observation period, in this study the mean creatinine clearance of diabetic patients was lower than that of non-diabetic patients. At no time did diabetic patients have a higher creatinine clearance and, presumably, therefore, did not have hyperfiltration commonly associated with diabetics. This is a remarkable difference from the Type I diabetic patients before the clinical onset of diabetic nephropathy. Since most diabetic renal transplant patients have poor glycemic control after kidney transplantation, the lack of apparent glomerular hyperfiltration in renal allografts is not due to better control of hyperglycemia. It is likely that all transplant kidneys, whether in diabetics or non-diabetics, have an increased glomerular filtration per functioning nephron, although the total glomerular filtration rate of the transplant kidney may not be high. It is conceivable that each functioning nephron of the transplant kidney in diabetic patients is already functioning at a maximum capacity, and thus the diabetic milieu may not elicit any further increase in glomerular filtration. The observation that diabetic patients had a lower creatinine clearance than non-diabetic patients in this study supports this notion. It is a conjecture that the lack of overt hyperfiltration in diabetic kidney transplant recipients might help delay a recurrence of diabetic nephropathy.
Virtually all transplanted kidneys from non-diabetic donors to diabetic patients reveal histologic changes of diabetic nephropathy within a few years after transplantation, but diabetic nephropathy rarely leads to graft failure at least in the first post-transplant decade28, 29, 30). Sixteen transplant kidney biopsies were performed at 2 to 7 years after transplantation for unexplained azotemia or proteinuria. Six of them showed thickened basement membranes, increased mesangeal matrix and glomerulosclerosis, consistent with early diabetic nephropathy. Recurrence of clinical diabetic nephropathy that was significant enough to cause graft failure, was observed in only one case. The Minnesota group10) reported that none of 265 primary renal transplant recipients developed recurrence of clinical diabetic nephropathy in the first decade, while only two of 100 patients who had functioning grafts for more than a decade subsequently lost graft function solely due to recurrence of diabetic nephropathy. Our results also indicate that recurrence and progression of clinically significant diabetic nephropathy is rare. Genetic propensity, poor control of hyperglycemia and persistence of hypertension are all considered risk factors for the development of diabetic nephropathy. Since diabetic kidney transplant patients are already known to have a propensity for developing nephropathy, and most of these patients continue to have hypertension and hyperglycemia, it is rather surprising not to observe recurrence of diabetic nephropathy in the transplant kidney more often and earlier. The absence of overt hyperfiltration in the transplant kidney may be one of the reasons for this observation. Other mechanisms, such as limited renal vasodilatation due to cyclosporine, also might help prevent the recurrence of diabetic nephropathy. The lack of genetic propensity in diabetic nephropathy within the donor’s kidney may also play a role.
In summary, we studied the long-term prognosis of Type I diabetic kidney transplant recipients and compared them with matched non-diabetic patients. We found patient survival rate of diabetic transplant recipients significantly lower than that of non-diabetic patients. Graft survival rate is, however, comparable to that seen in non-diabetic patients. Since a considerable number of patients with functioning grafts died from complications of cardiovascular disease, careful and aggressive intervention to modify cardiovascular risk factors should help improve both patient and graft survival in diabetic transplant recipients. Creatinine clearances of diabetic patients were lower than that of non-diabetic patients over the 10 years of observation period. Recurrence of clinical diabetic nephropathy in the transplant kidney was rare in the first decade of transplant, despite the fact that most risk factors for its recurrence continue to exist. Mechanisms for the absence of overt glomerular hyperfiltration and the rarity of recurrence remain to be elucidated.


