S-1 Monotherapy as a Neoadjuvant Treatment for Locally Advanced Gastric Cancer
Article information
Abstract
S-1, a novel oral fluoropyrimidine, is an effective therapeutic agent for gastric cancer. Herein, we report a case with locally advanced gastric cancer that achieved a curative resection after S-1 monotherapy as neoadjuvant treatment. A 68-year-old man was diagnosed with gastric cancer and massive lymphadenopathy involving the perigastric, celiac axis and splenic hilum. His clinical stage was cT3N2H0P0M0. Considering his relatively poor performance (ECOG 2, severe weight loss) and advanced age, we started the patient on S-1 monotherapy at a dose of 35 mg/m2 bid for 4 consecutive weeks followed by a 2-week rest. Follow-up study after 4 treatment cycles revealed disappearance of the lymphadenopathy of the perigastric and celiac axis with diminished extension of the stomach mass. The patient had a partial response (PR) with a 72% tumor reduction, according to the Response Evaluation Criteria in Solid Tumors (RECIST). His performance status was improved to an ECOG 1 and he gained 7 kg. A curative (R0) resection was achieved with a radical total gastrectomy and D2 dissection. The pathological stage was pT3N2M0, stage IIIB. In conclusion, S-1 neoadjuvant chemotherapy aided in the treatment of gastric cancer in this patient.
INTRODUCTION
Surgery is the main treatment strategy for gastric cancer; only R0 resection anticipates a potential cure. The prognosis of initially unresectable gastric cancer is poor; approximately 50% of patients have recurrent disease after a curative resection. This suggests that micrometastases are already present, in many cases, at the time of surgery1). Neoadjuvant chemotherapy has been attempted for the treatment of gastric cancers in patients with advanced T and N stage disease for the purpose of downstaging, improving resectability and survival2). Several neoadjuvant trials have been reported to provide such benefits. Several regimens containing cisplatin, anthracyclines and methotrexate are the main chemotherapeutic options. These regimens have demonstrated a response rate (RR) of 3 4~69% and a resectability of 36~60%, with a median survival of 16~28 months for initially unresectable or stage III-IV cases. However, in 19~33% of patients, the associated adverse effects of up to grade 3-4 have impeded the use of these agents3-6). Therefore, the selection of patients with a good performance status is the first consideration for effective neoadjuvant chemotherapy.
S-1 is a fourth-generation oral fluoropyrimidine based on the combination of tegafur with two biochemical modulators, 5-chloro-2,4-dihydroxypyridine (CDHP) and potassium oxonate (Oxo). S-1 mimics the protracted continuous infusion of 5-fluorouracil (5-FU) with enhanced efficacy and safety7). One earlier and two more current, phase II trials with monotherapy for advanced gastric cancer, in Japan, achieved a remarkable RR of 45% and 54% respectively. These results are consistent with the commonly used combination regimens containing cisplatin or anthracyclines8, 9). S-1 is also known to have less toxicity than other active agents in the treatment of gastric cancer. Nevertheless, there are only a few reports evaluating its efficacy for neoadjuvant treatment. In retrospective analyses, S-1 in combination with cisplatin achieved a RR of 44% and 79% and a downstaging rate of 47% and 85%, respectively10, 11). Furthermore, S-1 monotherapy as neoadjuvant treatment has recently been reported to induce a pathologically complete response (CR)12, 13) and curative resection. Based on these data, we report herein S-1 monotherapy as neoadjuvant treatment in a patient with locally advanced gastric cancer and poor performance status who achieved a curative resection.
CASE REPORT
A 68-year-old man with epigastric pain, anorexia and general weakness for 6 months was admitted to our hospital. He lost 9 kg in the last 6 months (12% loss). There was neither history of pre-existing chronic disease nor a family history of gastric cancer. His performance status was 2 according to the criteria of the Eastern Cooperative Oncology Group (ECOG).
Endoscopic examination demonstrated a diffuse-infiltrative lesion along the great curvature of the upper body of the stomach; the biopsy confirmed a moderately differentiated adenocarcinoma (Figure 1A, 1B). Abdominal-pelvic computed tomography (CT) showed a gastric cancer with massive lymph node enlargement in the perigastric area, upper retroperitoneum close to the celiac axis and the splenic hilum with no evidence of distant or peritoneal metastases (Figure 1C, 1D, 1E). The clinical stage of the patient was cT3N2H0P0M0 according to the staging system of the Japanese Gastric Cancer Association (JGCA).
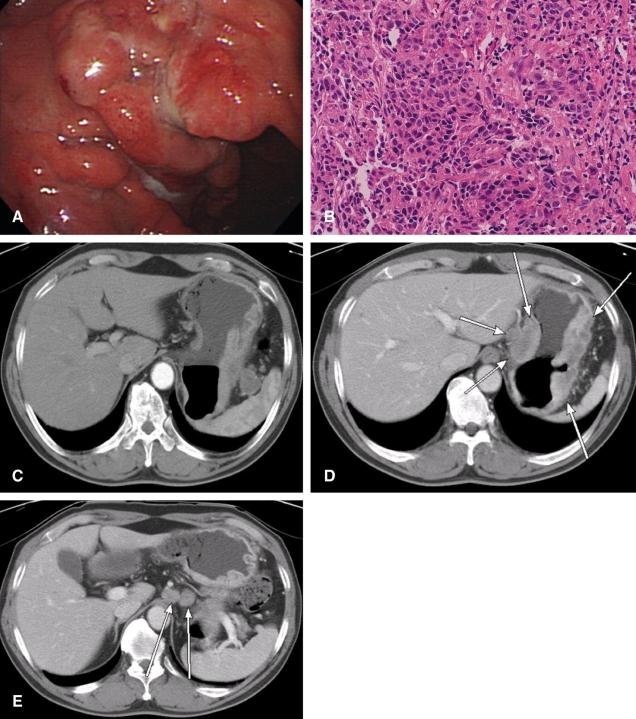
Initial endoscopic and radiological findings, (A) Endoscopy demonstrating a diffuse-infiltrative lesion at the great curvature of the upper body of the stomach. (B) Microscopic findings of adenocarcinoma with poor lumen formation (H&E, ×400). (C-E) Abdominal-pelvic computed tomography (CT) showing a malignant mass in the stomach (arrow) (D), and multiple metastatic lymph nodes in the perigastric, upper retroperitoneum close to celiac axis (long arrow) (E), and splenic hilum (thick arrow) (C).
Neoadjuvant chemotherapy was considered for this patient because of the advanced nodal involvement. However, significant weight loss, advanced age and a relatively poor general condition prevented full-dose administration of a multi-drug systemic chemotherapy regimen. Alternately, we planned S-1 monotherapy at a dose of 35 mg/m2 twice a day for 4 consecutive weeks followed by a 2-week resting period.
Follow-up endoscopy after 4 cycles showed a decrease in the extent of infiltrative lesions of the stomach (Figure 2A). The CT scan demonstrated the disappearance of lymph node enlargement around the perigastric area, celiac axis and also a decrease in the size of the splenic hilar lymph nodes along with a reduction in the stomach mass (Figure 2B, 2C, 2D). According to the RECIST criteria, a PR with a 72% decrease of the target lesions (sum of target lesion, 90 mm to 26 mm) was achieved. During the 4 cycles of treatment, there was no treatment delay or dose reduction due to adverse events. The patient had grade 1 fatigue and nausea and grade 2 skin rashes without any grade 3-4 toxicity. The performance status improved to ECOG 1 after the administration of S-1 and the patient gained 7 kg of body weight. The diagnostic laparoscopy showed a serosa-involving lesion on the upper body of the stomach extending from the anterior to the posterior wall with severe adhesions and surrounding tissues.
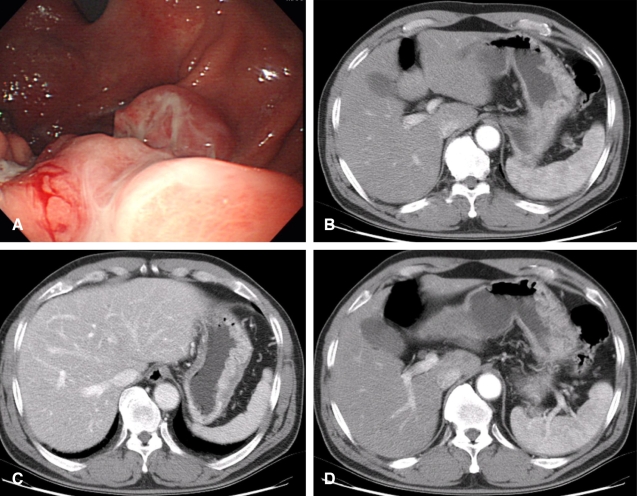
Follow-up endoscopic and radiological findings after 4 cycles of S-1 treatment. (A) Endoscopy demonstrating a reduced stomach mass. (B-D) Abdominal-pelvic CT showing disappearance of the lymph node enlargement around the perigastric area and celiacaxis (D), and also diminished splenic hilar lymph nodes (long arrow) (B) along with a decreased stomach mass (C).
A radical total gastrectomy with D2 lymph node dissection and combined splenectomy were performed. Macroscopically, the excised specimen showed a diffuse infiltrative lesion with serosal surface exposure (se) measuring 180×130 mm. Microscopically, there was transmural involvement of the tumor (Figure 3). Metastatic adenocarcinoma in 12 out of 65 resected lymph nodes was noted. An adequate resection margin was obtained on both ends and there was no microscopic involvement at the margins identified. According to Becker's criteria, more than 50% of the tumor bed was composed of tumor cells; therefore, the pathological response was defined as minor14). The final pathological stage was pT3N2M0 (H0P0), Stage IIIB, according to both the JGCA and the American Joint Committee on Cancer (AJCC) systems. The postoperative CT scan showed no evidence of residual disease. After an additional 3 cycles of S-1 monotherapy were administered as adjuvant chemotherapy, the patient is without any evidence of recurrence after 6 months of follow-up.
DISCUSSION
Neoadjuvant chemotherapy for locally advanced gastric cancer has several advantages. Early initiation of systemic therapy may result in disease downstaging, eliminate micrometastases and enable a curative resection. Furthermore, neoadjuvant chemotherapy provides prognostic information due to the in vivo chemosensitivity testing. However, there are also potential risks involved. By delaying definitive local therapy, treatment-resistant clones can emerge during therapy. In addition, considerable tumor shrinkage is required in a short period for the chemotherapeutic regimen to obtain effective downstaging with the neoadjuvant treatment. However, regimens with a high tumor response are usually associated with substantial toxicity. Therefore, neoadjuvant therapy may not be feasible in some circumstances such as in patients with an unfavorable performance status or advance age. In the clinical trials using cisplatin or anthracycline-based regimens, although downstaging was attained, only 20~54% of the patients completed the planned treatment. As many as one-third of the patients experienced grade 3-4 adverse events3-6).
Therefore, novel chemotherapeutic agents such as taxanes, irinotecan and oral fluoropyrimidines might be candidates for future neoadjuvant trials with effects on tumor shrinkage comparable to and more favorable toxicity profiles than traditional agents.
Among the above cited agents, S-1 might be a useful treatment option because of the results of several phase II trials on advanced gastric cancer. There are a few reports on S-1 in the neoadjuvant setting. Two Japanese retrospective analyses of S-1, in combination with cisplatin as neoadjuvant treatment in stage III-IV disease, achieved a RR of 44% and 79% and a downstaging rate of 47% and 85%, respectively. However, these high responses are mainly due to a high PR not a pathological CR possibly implicating a survival benefit10, 11). Recently, preoperative administration of S-1 alone has been shown to induce favorable responses in patients in poor clinical condition. The total disappearance of peritoneal dissemination was observed with S-1 and cisplatin, and there was a pathological CR in a 78-year old patient who was treated with S-1 alone12). Our selection of S-1 in the present case was based on these findings. Our patient was not a candidate for full-dose multi-drug systemic chemotherapy due to his poor health status. Although downstaging was not observed in our case, probably due to a very large initial tumor burden, there was marked resolution of the massive lymphadenopathy around the celiac axis. This is consistent with other phase II studies on S-1 monotherapy that reported a higher efficacy for the abdominal lymph nodes (75%) compared to the primary gastric mass (28%) or visceral organs (30%)9).
Another aspect of neoadjuvant chemotherapy to consider is safety, which in turn is associated with treatment compliance. In this respect, S-1 has an advantage with favorable toxicity profiles compared to other agents. Neutropenia and anemia were the main adverse events reported in Asian patients9, 15) and the neoadjuvant combination with cisplatin showed only 7% of cases with grade 3-4 adverse events10, 11). Our patient finished the treatment without significant toxicity or dose reduction. Moreover, the patient reported a sense of well being, had an improved appetite and gained weight. Therefore, S-1 monotherapy was safe and effective treatment for this patient and might be considered in the future as neoadjuvant treatment for gastric cancer. In conclusion, the results of this case study suggest that S-1 might be a safe and effective treatment option for neoadjuvant chemotherapy in locally advanced gastric cancer, especially when accompanied byperigastric lymphadenopathy and a poor general health status in patients of advanced age. The role of S-1 in the neoadjuvant setting for the treatment of gastric cancer remains to be established by clinical trials.
Notes
This work was supported by a grant from Korea Science and Engineering Foundation (KOSEF) through the Cancer Metastasis Research Center at Yonsei University, College of Medicine.
