Effects of vitamin D supplements in patients with chronic hepatitis C: a randomized, multi-center, open label study
Article information
Abstract
Background/Aims
We aimed to assess the role of vitamin D supplementation in the response to pegylated interferon-α (PEG-IFN-α) plus ribavirin (RBV) treatment in patients with chronic hepatitis C (CHC).
Methods
Our study was a multi-center, randomized controlled trial in 11 hospitals. CHC patients were randomly assigned (1:1) to two groups namely, PEGIFN-α plus RBV (control group) or PEG-IFN-α plus RBV + vitamin D (800 IU daily) (vitamin D group). The primary end-point was the rate of sustained virologic response (SVR).
Results
One hundred forty eight CHC patients were randomly assigned to two groups. Seventy-one patients received the PEG-IFN-α plus RBV and 77 patients received the PEG-IFN-α plus RBV + vitamin D. A total of 105 patients completed the study (control group, 47 vs. vitamin D group, 58). Baseline characteristics were mostly similar in both the groups. There was a modest but non-significant increase in SVR in the vitamin D group compared to the control group with the intention to treat analysis (64.0% vs. 49.3 %, p = 0.071) as well as in the per protocol analysis (control group vs. vitamin D group: 74.5% vs. 84.5%, p = 0.202). Fifty-two patients (73.2%) in the control group and 63 patients (81.8%) in the vitamin D group experienced at least one adverse event. The drop-out rate due to adverse effects was not different between both groups (control group vs. vitamin D group: 19.7% vs. 10.4%, p = 0.111).
Conclusions
Vitamin D supplement did not increase SVR in treatment naïve patients with CHC irrespective of genotype.
INTRODUCTION
Vitamin D has a variety of functions in addition to calcium and bone metabolism, such as insulin resistance, metabolic diseases, immune function and various functions with regard to muscles. Vitamin D is a seco-steroid hormone involved in innate immunity, cell differentiation, inflammation and fibrogenesis besides regulation of bones and calcium/phosphorus homeostasis [1]. Vitamin D deficiency is known to increase the risk of cancer, autoimmune diseases, cardiovascular diseases and infectious diseases [2-5].
The institute of medicine and the World Health Organization define the normal range of vitamin D as above 20 ng/mL [6]. Prevalence of vitamin D insufficiency is relatively common, and is about 52% to 72% in the average risk group [7-9]. The prevalence of vitamin D insufficiency in the general population was 41.6% in the United States [10], 40.4% in Europe [11], and 40.8% in Japan [12]. Known prevalence of vitamin D insufficiency in chronic liver diseases is about 64% to 94% [13]. Previous meta-analysis for patients with chronic hepatitis C (CHC) reported that the prevalence of vitamin D insufficiency was about 80%, much higher than that in the general population [14]. In CHC, the lower the levels of vitamin D, the greater is the degree of fibrosis and inflammation in the liver, and the poorer is the response to interferon-based treatment [14,15].
Although there is clear association between chronic liver disease and vitamin D insufficiency, it is not clear about the mechanism of serum vitamin D insufficiency in chronic liver disease. At the same time, there is another controversy as to whether low serum vitamin D causes a decrease in immune function resulting in high prevalence of viral hepatitis or chronic liver diseases lead to vitamin D metabolic disorders in liver to induce vitamin D deficiency [16-18].
For these reasons, many studies have examined the effects of vitamin D supplements on sustained virologic response (SVR) in pegylated interferon-α (PEG-IFN-α) plus ribavirin (RBV) therapy in patients with CHC [19-23]. Several studies have been reported on the association of vitamin D supplementation with treatment response in patients with CHC [19-21]. However, a possible role of vitamin D supplementation in PEG-IFN-α plus RBV therapy is still debatable [24]. We investigated to assess the role of vitamin D supplementation in the response to PEG-IFN-α plus RBV treatment in naïve patients with CHC.
METHODS
Patients
Our study was a randomized, multi-center, open-label trial between September 2011 and April 2015 in 11 hospitals in the Republic of Korea. Patients with CHC were screened in this study. A diagnosis of CHC was established according to the guidelines of the Korea Association for the Study of the Liver (KASL) [25]. The inclusion criteria were age 20 to 75 years, patients found positive in hepatitis C virus-ribonucleic acid polymerase chain reaction (HCV-RNA PCR) screening, normal serum calcium level before treatment, and HCV genotypes 1, 2, and 3. Exclusion criteria were hepatocelluar carcinoma (HCC) at enrollment or past history of HCC within last 1 year, decompensated cirrhosis (Child-Pugh class B or C), absolute neutrophil count < 1,000/mm3 or platelet count < 70,000/mm3, serum creatinine level above 1.5 times to upper normal limit, a present or past history of severe psychiatric diseases, parathyroid disease, uncontrolled thyroid disease, co-infection with other hepatitis virus or human immunodeficiency virus, history of malignant diseases besides HCC within last 2 years, patients who were considered unfit to perform clinical trial, and pregnancy. This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committee of each hospital (for example, IRB No. of Hanyang University Hospital: 2011-05-001) and is registered at ClinicalTrial.gov (NCT01439776).
After applying inclusion and exclusion criteria, they were stratified according to HCV viral load (≥ 600,000 IU/mL vs. < 600,000 IU/mL) and genotypes (1 vs. 2 and 3), and randomly assigned (1:1) to two groups namely, PEGIFN-α plus RBV (control group) and PEG-IFN-α plus RBV + vitamin D (vitamin D group). All the included patients had provided written informed consent.
Treatment strategy
Treatment of CHC was based on the guidelines of the KASL [25]. Control group received once a week PEG-IFN α-2a 180 μg (Pegasys, Roche Ltd., Basel, Switzerland) plus oral ribavirin 1,000 mg (body weight < 75 kg) or 1,200 mg (body weight ≥ 75 kg) daily in genotype 1, or oral ribavirin 800 mg daily in genotype 2 and 3. Vitamin D group received identical therapy with oral vitamin D 800 IU (GNC Korea, Seoul, Korea) daily. The planned duration of treatment was 48 weeks for genotype 1 and 24 weeks for genotypes 2 and 3.
When the absolute neutrophil count fell below 750/mm3 or platelet count fell below 50,000/mm3, the PEGINF-α dose was decreased, and when the absolute neutrophil count fell below 500/mm3 or platelet count fell below 25,000/mm3, the treatment was temporarily stopped until absolute neutrophil count became more than 1,000/mm3 or platelet count became more than 75,000/mm3. When resuming PEG-INF-α administration, a starting dose of 90 μg was used and neutrophil count or platelet count was closely monitored. The ribavirin dose was adjusted considering the following. (1) When the hemoglobin level decreased below 10 g/dL, the ribavirin dose was gradually decreased. When the hemoglobin level decreased below 8 g/dL, the ribavirin administration was stopped. (2) Patients who discontinued ribavirin therapy continued to receive PEG-INF-α monotherapy for the remaining treatment period. Once the anemia was restored, the ribavirin was re-administered according to the clinician's decision.
Monitoring and follow-up
Patients visited the clinic at baseline, at every 4 weeks for the first 12 weeks of treatment and then every 3 months until the completion of treatment. All patients followed up at 24 weeks after the completion of treatment. At each visit, a physical examination was conducted, adverse drug events were recorded, and compliance with medication intake was assessed by pill counts. Body mass index, vital signs, and blood tests such as complete blood count, prothrombin time (international normalized ratio), alanine aminotransferase, aspartate aminotransferase, total bilirubin, albumin, creatinine, calcium, phosphate, glucose, lipid profiles (total cholesterol, triglycerides, high density lipoprotein cholesterol, low density lipoprotein cholesterol), fasting insulin and 25-hydroxy (25[OH]) vitamin D3 were performed at baseline. Vitamin D deficiency was defined as 25(OH) vitamin D3 < 10 ng/mL and vitamin D insufficiency as 25(OH) vitamin D3 of ≥ 10 to < 20 ng/mL. Patients were screened for hepatocellular carcinoma every 6 months by α-fetoprotein assay and ultrasonography.
Serum HCV RNA quantification was measured using real-time polymerase chain reaction. HCV RNA was performed at baseline and after 4, 12, and 48 weeks of treatment for genotype 1 and at baseline and after 4, 12, and 24 weeks of treatment for genotype 2 and 3. Rapid virological response, early virological response and end of treatment response were defined as the undetectable HCV RNA at 4 weeks after initiation of treatment, as the undetectable HCV RNA or reduction by more than 2 log10 copies/mL at 12 weeks of treatment, and as the undetectable HCV RNA at the end of treatment, respectively. SVR was defined as the undetectable HCV RNA at 24 weeks after the completion of treatment and was considered as cure.
Endpoints and safety evaluation
The primary endpoint was the rate of SVR. Secondary endpoints were risk factors for SVR. The responders were defined as patients who achieved SVR, and the non-responders were defined as those who did not achieve SVR. Safety assessments included adverse events, laboratory findings and vital signs. Adverse events were classified as mild, moderate or severe, and as definitely related, probably related, possibly related, probably not related or definitely not related.
Statistical analysis
The level of significance for the primary endpoint was 2.5% on the single side because this study is a superiority trial. The 91 subjects in each group provided a power of 80% for identifying SVR of 77% in PEG-IFN-α/RBV treatment and a difference of 15% in SVR between control group and vitamin D group. Assuming a 20% dropout rate, a sample size of 114 subjects in each group was needed. However, this study was stopped early because of the difficulty in recruiting patients with the advent of new oral directly acting agents for treatment of CHC.
Categorical variables are given as frequencies (%) and continuous variables are given as mean values with standard deviations. Clinical and laboratory factors that affected SVR were analyzed by using chi-square test or the Fisher exact test. Subsequently, multivariate logistic regression analysis was performed to assess the factors that showed significant associations with SVR in the univariate analyses. Statistical significance in 2-sided tests was defined as p < 0.05. All statistical analyses were performed by using the SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics
Seventy-one patients received the PEG-IFN-α plus RBV and 77 patients received the PEG-IFN-α plus RBV + vitamin D. Twenty-four patients (33.8%) in the control group and 19 patients (24.7%) in the vitamin D group, dropped out during the treatment period (Fig. 1). There was no significant difference in drop-out rates between the two groups (p = 0.222). Baseline characteristics were mostly similar in both the groups, as shown in Table 1.
Viral response rate
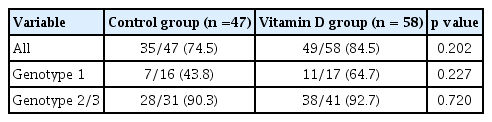
Vitamin D group (48/75, 64.0%) showed non-significantly higher the rates of SVR as compared to the control group (36/73, 49.3%) (p = 0.071) (Fig. 2) in the intention to treat analysis. The subgroup analysis according to genotype yielded similar results for the rates of SVR in both the groups (for genotype 1, control group: 26.7% vs. vitamin D group: 43.5%, p = 0.200; and for genotype 2 and 3, control group: 65.1% vs. vitamin D group: 73.1%, p = 0.402). SVR was 74.5% (35/47) in the control group and 84.5% (49/58) in the vitamin D group, but the difference between both the groups was not statistically significant (p = 0.202) using per protocol (PP) analysis (Table 2).
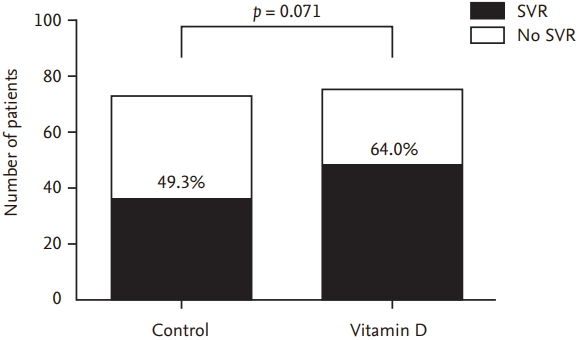
Sustained virologic response (SVR) between control group and vitamin D group in intention to treat analysis.
Factors associated with SVR
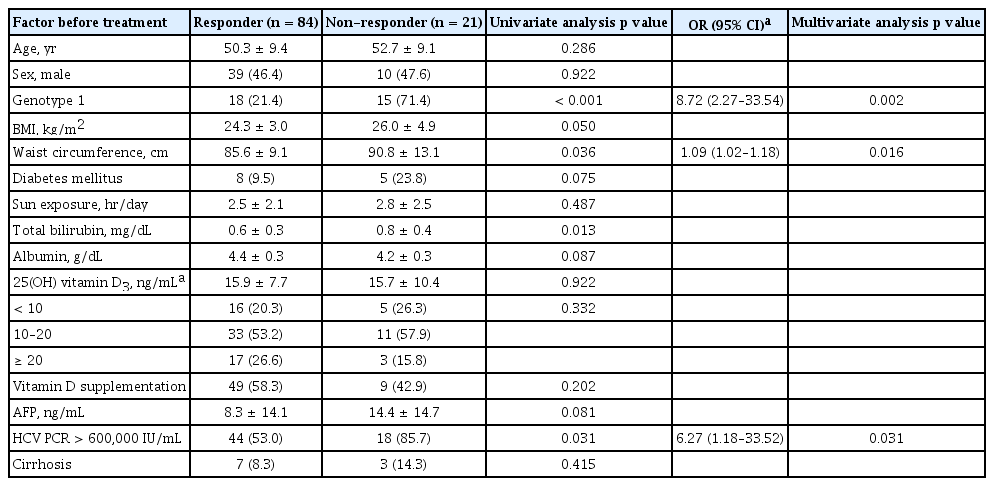
Of the 105 patients in the PP analysis, 21 patients (20.0%) failed to achieve SVR. In univariate analysis, the failure to achieve SVR was significantly associated with genotype 1, high viral load (> 600,000 IU/mL), waist circumference and total bilirubin (Table 3). In multivariate analysis, genotype 1, high viral load (> 600,000 IU/mL) and waist circumference were independent predictors of the failure to achieve SVR (Table 3). Baseline serum vitamin D levels and additional supplementation of vitamin D were not associated with viral response.
Viral response rate according to serum vitamin D level.
Baseline serum vitamin D levels were 15.6 ng/dL in the control group and 15.5 ng/dL in the vitamin D group (p = 0.809). Vitamin D deficiency was observed in 18 patients (26.9%) in the control group and 19 patients (25.7%) in the vitamin D group (p = 0.965). SVR rate did not vary according to baseline serum vitamin D levels (p = 0.161). Also, in the subgroup analysis of patients with 25(OH) vitamin D3 < 20 ng/mL, there was no difference in SVR rate according to vitamin D supplementation (all p value = 1.000) (Table 4).
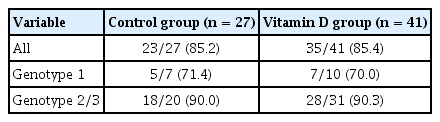
Sustained virologic response between control group and vitamin D group in patients with 25(OH) vitamin D3 < 20 ng/mL
In the vitamin D group (n = 58), the mean serum vitamin D levels increased from 17.0 ± 8.8 ng/mL before treatment to 17.6 ± 9.0 ng/mL after treatment, but there was no statistical difference (p = 0.524). There were 26 patients (44.8%) with elevated serum vitamin D levels and 32 (55.2%) with decreased serum vitamin D levels after treatment. SVR rate did not vary according to the change in vitamin D levels (group with decreased serum vitamin D levels vs. group with elevated serum vitamin D levels: 80.8% vs. 90.6%, p = 0.446).
Safety
A total of 115 patients (77.7%) experienced at least one adverse event, 52 (73.2%) of them in the control group and 63 (81.8%) in the vitamin D group. Most adverse events were mild and similar in both the groups. Serious adverse events occurred in 10 patients, but all recovered. The drop-out rate due to adverse effects was not different between both the groups (control group vs. vitamin D group: 19.7% vs. 10.4%, p = 0.111). There was no adverse event related to vitamin D supplements.
DISCUSSION
In this study, we evaluated the efficacy and safety of vitamin D supplementation in the response to PEG-IFN-α plus RBV therapy in treatment of naïve patients with CHC. Vitamin D supplement did not significantly increase SVR in PEG-IFN-α treatment of naïve patients with CHC regardless of genotype. Also, baseline serum vitamin D levels were not associated with treatment response.
It is well known that serum vitamin D levels are decreased in chronic liver diseases [26]. In this study, 75.2% of enrolled patients with CHC had vitamin D insufficiency and deficiency. In addition, the lower serum vitamin D levels in patients with CHC showed a more advanced degree of fibrosis and a lower treatment response to IFN-based therapy [14,15]. Therefore, there have been many studies on whether vitamin D supplementation positively affects treatment response (SVR) in patients with CHC who have been treated with PEGIFN-α [19-22].
It is still unclear how vitamin D supplementation has a synergistic effect on the improvement of the viral response. Several mechanisms have been suggested for this effect. In CHC, various proinflammatory cytokines such as TNF-α and chemokine CXCL10 cause intrahepatic inflammation and disease progression [27,28]. Vitamin D can have an antiinflammatory effect on these cytokines. Vitamin D also has antifibrotic effects. Abramovitch et al. [29] showed that in vitro and in vivo 1,25(OH)2D inhibits fibrosis by inhibiting the proliferation and profibrosis action of hepatic stellate cells. Recently, Komolmit et al. [30] showed that vitamin D supplementation in patients with CHC improved the serum markers such as TGF-β, TIMP-1, MMP2, and MMP2, associated with hepatic fibrogenesis. This suggests that vitamin D plays an important role in the reversal of hepatic fibrosis [30]. In addition, in vitro, vitamin D enhances the expression of IFN-β and the induction of IFN-stimulated genes resulting in a synergic effect on the antiviral activity of IFN [31]. Vitamin D also affects the improvement of insulin resistance, which affects SVR [32].
This study is a randomized, multi-center, open-label trial. In this study, serum vitamin D levels did not increase viral response in patients with CHC. There were some reports whether vitamin D supplement can augment viral response in patients with CHC [19-23]. Although there have been some studies whether vitamin D use can be helpful in the treatment of CHC infection [19-21], the results were controversial. Abu-Mouch et al. [20] reported in a study of 40 patients with HCV genotype 1 that, vitamin D 1,000 IU/day supplementation resulted in a 40% increase in SVR rates compared to standard therapy. Same group also published that adding vitamin D to conventional PEG-IFN treatment for patients with HCV genotype 2 and 3 increased viral response (control group vs. vitamin D group: 95% vs. 77%, p < 0.001) [21]. However, these reports were not support by following studies. Yokoyama et al. [22] added vitamin D (1,000 IU/day) to PEG-IFN/RBV therapy in patients with HCV genotype 1b; however, SVR rates were not different in the vitamin D and control groups (64.3% vs 50%, p = 0.19). Esmat et al. [23] also tried to show the possible role of vitamin D supplementation in patients with HCV genotype 4. However, the study by Esmat et al. [23] showed that vitamin D supplementation did not increase SVR in patients with HCV genotype 4. Therefore, there is still controversy whether vitamin D supplement can increase viral response. Recently, meta-analysis reported that vitamin D supplementation improved SVR in patients with CHC [33]. However, as noted in this meta-analysis, it is difficult to confirm this efficacy of vitamin D supplementation, because most studies have small sample sizes, low methodological quality and heterogenicity [33]. Another reason of this difference seems to result from higher SVR in Asia, especially in East Asia than in Western countries, because SVR rate of IFN-based treatment was quite different according to geographic region [33].
There have been many reports that serum vitamin D levels were associated with treatment success before starting the treatment [14,15], but there is a lack of research into whether serum vitamin D levels increase after successful treatment. Lange et al. [34] pointed out that HCV infection itself affects vitamin D metabolism and synthesis. In patients who achieved SVR, serum vitamin D levels were slightly elevated (pretreatment vs. posttreatment: 16.2 ng/mL vs. 18.2 ng/mL) and incidence of vitamin D deficiency (< 10 ng/mL) was lower (pretreatment vs. posttreatment: 33% vs. 26%) in that study, but it was not statistically significant. At the same time, seasonal and dietary factors were also not considered. A recent cohort study of 218 patients reported that serum vitamin D levels remained unchanged during direct-acting antiviral therapy (pre-treatment vs. post-treatment: 25.3 ± 15.9 vs. 26.4 ± 12.5, p = 0.10) [35]. In addition, this cohort study did not consider important variables such as seasonal factors [35].
Serum vitamin D levels are generally more affected by sun exposure than food and supplements [1]. However, these are now important sources, especially in modern urban people who live indoors [1]. In several studies, vitamin D supplementation increased serum vitamin D levels [19-23]. In this study, serum vitamin D levels increased after supplementation but were not statistically significant. Therefore, it is thought that serum vitamin D level is influenced not only by supplements but also by various factors. In addition, this seems to be partially due to the seasonal differences caused by 33 to 38 degrees latitude of Korea. In a recent study, although vitamin D intake was reduced, vitamin D levels were increased by weight loss in patients with nonalcoholic fatty liver disease. This suggests that weight loss has a more influence on serum vitamin D levels than vitamin D intake [36].
Taken together these facts, the causal relationship of lack of vitamin D level in chronic liver disease is still unclear. The results of this study might suggest that insight into the causal relationship of lack of vitamin D concentration in patients with CHC. In this study, additional supplementation of vitamin D in patients with CHC did not increase SVR rate, nor was there any association with baseline vitamin D levels and SVR rate. Also, vitamin D levels did not increase after successful hepatitis C treatment. These results suggest that the reduction of vitamin D level in CHC is more likely to be caused by other factors such as bad life style rather than chronic liver disease itself. Therefore, it may be more important to find other risk factors that may lower vitamin D levels than vitamin D supplementation for the correction of low vitamin D levels in patients with chronic liver disease.
This study had several limitations. First, this prospective and randomized study was open label but not placebo controlled. Therefore, the patients knew whether vitamin D was administered or not. Second, during the study period, this study was early terminated by the advent of direct acting antivirals and only 64.9% of the target patients were registered. Therefore, a clear association between vitamin D supplement and SVR has not been elucidated. Third, the degree of ultraviolet exposure and weight changes that could affect serum vitamin D levels were not assessed. Finally, there is no data on hepatic fibrosis and IL-28B polymorphism, important factors affecting SVR in PEG-IFN-α plus RBV therapy.
In conclusion, vitamin D supplementation did not increase SVR in PEG-IFN-α plus RBV treatment of naïve patients with CHC regardless of genotype. At the same time, serum vitamin D levels were not associated with treatment response. Therefore, larger, randomized, placebo-controlled trial is needed to assess a robust association between vitamin D supplement and SVR in patients with CHC.
KEY MESSAGE
1. Vitamin D supplementation did not increase sustained virologic response in pegylated interferon-α and ribavirin treatment of naïve patients with chronic viral hepatitis C regardless of genotype.
2. Also, serum vitamin D levels were not associated with treatment response.
Notes
No potential conflict of interest relevant to this article was reported.