Proton pump inhibitor use is associated with hip fracture development: a nationwide population-based cohort study
Article information
Abstract
Background/Aims
Effect of proton pump inhibitor (PPI) use on the risk of hip fracture is controversial. This study aimed to clarify the association between PPI use and hip fracture risk using a large cohort.
Methods
This study recruited participants from the nationwide cohort (n = 1,025,340). After exclusion of participants who had hip fractures or were aged less than 40 years during the baseline period (2002 to 2004), 371,806 participants were followed to 2013. Participants prescribed PPIs for more than 90 days during baseline period were defined as users. Fracture cases were defined when participants were hospitalized with claims of a hip fracture.
Results
During 4,159,343 person-years of follow-up, fractures developed more often in PPI users than in nonusers (relative risk [RR], 1.787; 95% confidence interval [CI], 1.260 to 2.534; p = 0.002). The results persisted after adjusting for age, sex, and many drugs relevant to osteoporosis or influential in bone health. Furthermore, fracture risk associated with PPI use increased with duration of use (p trend < 0.001). The fully adjusted RRs of hip fracture development were 1.350 (95% CI, 1.203 to 1.515) for 1- to 90-day users, 1.487 (95% CI, 0.957 to 2.311) for 91- to 180-day users, and 1.771 (95% CI, 0.931 to 3.368) for > 180-day users. The positive association between PPI use and fracture was also confirmed in a subgroup with health screening data where further adjustment for body mass index, smoking status, alcohol consumption, and physical activity was available (adjusted RR, 2.025; 95% CI, 1.151 to 3.564, p = 0.014).
Conclusions
PPI use is associated with hip fracture development.
INTRODUCTION
Proton pump inhibitors (PPIs) are the most commonly used drugs worldwide, and their use has considerably increased over the last decade [1,2]. Owing to their potent acid suppression effect, PPIs are primarily used for gastroesophageal reflux disease (GERD) and peptic ulcer management [3-7]. Increasing prevalence of GERD and the need for peptic ulcer prophylaxis has led to a further increase in PPI use [8-12]. In addition, inappropriate prescriptions contribute to increased PPI use [13,14]. However, there are growing health-related concerns associated with PPI use-related potential adverse effects and the financial burden associated with extensive PPI use [15-22]. Indeed, several studies have suggested that PPI use increases the risk of fractures, especially of the hip [23-30].
One potential mechanism by which PPI use increases the risk of hip fracture is calcium malabsorption secondary to gastric acid suppression [31]. Decreased fractional calcium absorption is known to be associated with an increased risk for hip fracture [32]. However, observations from longitudinal studies examining the effects of PPI on bone mineral density (BMD) are contradictory [33,34]. A lack of biological plausibility could argue against the association between PPI use and hip fracture [35]. Thus, this study aimed to determine whether PPI use increases the risk of hip fracture and explore the interaction with osteoporosis in a large cohort, representative of the general population.
METHODS
Data source
The National Health Insurance Service (NHIS) is a single-insurer system that covers all citizens and maintains and stores national records for healthcare utilization and prescriptions in South Korea. The NHIS developed the National Health Information Database (NHID), containing personal information, demographics, and medical treatment data using participants’ medical bill expenses claimed by medical service providers for public health and medical research. Owing to the lack of confidentiality, the NHIS established a representative National Sample Cohort (NHIS-NSC) using the 2002 NHID. The NHIS-NSC comprised 1,025,340 participants (accounting for 2.2% of the total eligible Korean population in 2002) who were selected by systemic stratified random sampling, and followed up for 11 years until 2013 [36]. These data could be used with permission of NHIS.
Study population
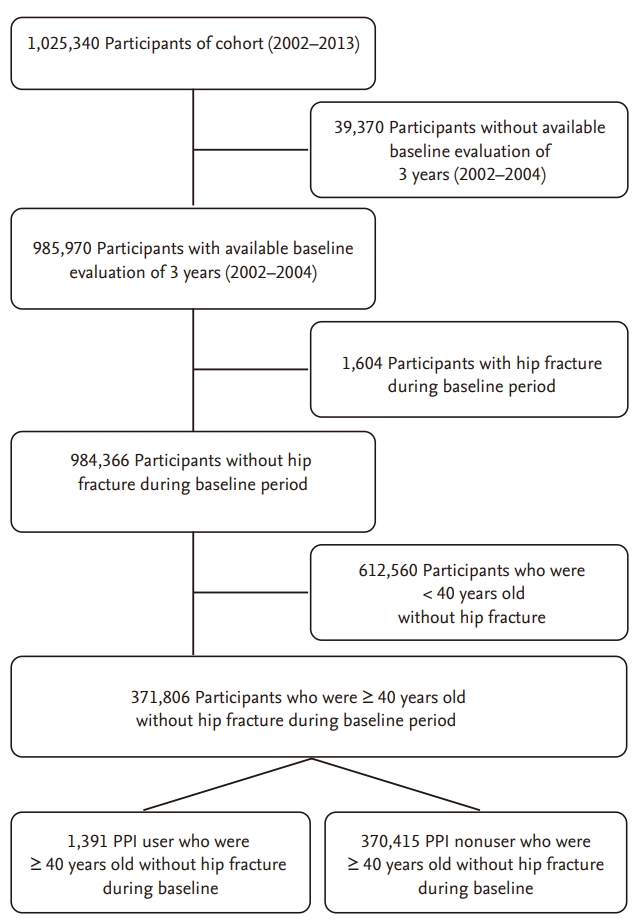
This study was conducted in a retrospective cohort design and recruited participants of the NHIS-NSC (n = 1,025,340). Participants were excluded from the study if they met the following criteria: (1) disqualification due to death or emigration during the baseline period between 2002 and 2004 (n = 39,370), (2) hip fractures during the baseline period (n = 1,604), or (3) age of less than 40 years in 2002 (n = 612,560). Finally, 1,391 PPI users and 370,415 PPI nonusers who were aged 40 years or more without hip fractures during the baseline period were included in the analysis (Fig. 1). The enrolled participants were followed up from 2005 to 2013 to identify participants who developed hip fractures. This study protocol was reviewed and approved by the Institutional Review Board of the Samsung Medical Center (2015-07-164) and the NHIS. All methods were performed in accordance with the relevant guidelines and regulations. The boards waived the requirement for informed consent.
Baseline exposure ascertainment
Individual durations of medications were determined according to the intended duration of each prescription recorded in the database. Participants who were prescribed PPIs or H2RAs for more than 90 days during the baseline period were included in the PPI or histamine 2-receptor antagonist (H2RA) user group, respectively, while the remaining were placed in the nonuser group. If participants had a period when both PPIs and H2RAs were prescribed, days of the period were applied to the duration of each drug. Regarding the use of other medications relating to bone health including bisphosphonate, calcium, vitamin D, hormone replacement treatment, steroid, thiazide, and anti-parathyroid hormone (anti-PTH), participants were classified into the user group when the duration of prescription was more than 30 days during the baseline period. The presence of osteoporosis was determined based on identification of the International Classification of Diseases, 10th revision (ICD-10) code M819 documented during the baseline period.
Among the study population, 131,689 participants had health screening data including body mass index (BMI), smoking status, alcohol consumption, and physical activity. BMI was calculated as weight in kilograms divided by height in meters squared. Smoking status was categorized into never or former and current smoker. Alcohol consumption was categorized into none, social (up to 2 days per week), or modest (more than 3 days per week). Physical activity was categorized into no exercise or regular exercise (at least every week).
Outcome ascertainment
Hip fracture cases were defined as those with claims for hip fracture (ICD-10 S72) from 2005 to 2013. The first date for the claims for hip fracture was used as the index date. To include definite fracture events, we excluded participants who were not hospitalized within 30 days from the index date.
Statistical analysis
Categorical variables were summarized with frequency and percentage, and were compared using the chisquare test. Because age information was originally provided as age groups (at an interval of every 5 years), we calculated age of a participant as the median age of the age group to which the participant belonged. Age was summarized as mean ± SD, and was compared using the independent t test.
Relative risk (RR) of hip fracture development with PPI use was crudely examined with the chi-square test, and then, further analyzed with adjustment using three multiple logistic regression models. Model 1 was adjusted for age group and sex. Model 2 was further adjusted for osteoporosis, use of bisphosphonate, use of calcium, use of vitamin D, and use of hormone replacement treatment, which is relevant to osteoporosis. Finally, model 3 was further adjusted for use of steroid, use of thiazide, and use of anti-PTH, which is influential in bone health. RR of hip fracture development with H2RA use was investigated in the same way. In addition, subgroup analyses were conducted for confounding factors, and interaction effect of each confounding factor and PPI use on hip fracture development was checked. Adjustment for multiple comparisons was not performed in this subgroup analyses.
In this study, we assumed that patients who were prescribed PPIs for more than 90 days during the baseline period were at a significant risk for hip fracture development. We conducted sensitivity analysis to assess the possibility that these assumptions had been violated. Thus, we examined the association between duration of PPI use and risk of hip fracture development, and subdivided subjects into four groups: PPI never-user (0 days), 1- to 90-day user, 91- to 180-day user, and > 180-day user. Cochran-Armitage trend test and multiple logistic regression models were used.
In a subgroup of 131,689 participants who had health screening data, RR of hip fracture development with PPI use was crudely examined, and was adjusted for age group, sex, BMI (< 25 or 25 kg/m2), smoking status, alcohol consumption, physical activity, osteoporosis, use of bisphosphonate, use of calcium, use of vitamin D, use of hormone replacement treatment, use of steroid, use of thiazide, and use of anti-PTH. Participants with any missing data were not included in this analysis.
Statistical significance was set at a p value of 0.05. Data processing and statistical analyses were conducted with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R: a language and environment for statistical computing version 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The baseline characteristics of the study population are shown in Table 1. The final analysis included 371,806 participants. The mean age ± SD of the participants was 54.3 ± 11.3 years, and 47.5% (n = 176,487) were men. The prevalence of PPI use during the baseline period was 0.37% (n = 1,391). Among them, gastric ulcer (62.4%) was the most common indication for PPI use followed by GERD (55.6%) and duodenal ulcer (24.3%). Compared to PPI nonusers, PPI users were significantly older and more likely to be men, and to use bisphosphonate, calcium, vitamin D, hormone replacement treatment, steroid, and thiazide.

Baseline characteristics of 371,806 participants who were aged 40 years or more without hip fracture according to use of PPI during baseline period (2002 to 2004)
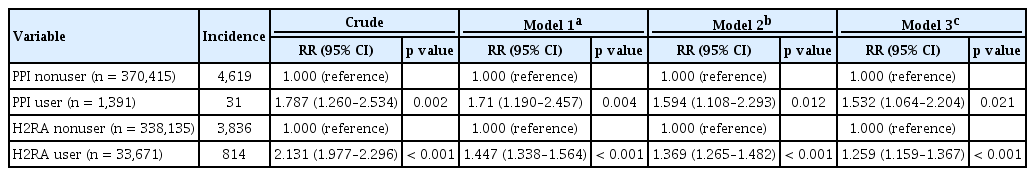
During 4,159,343 person-years of follow-up, hip fractures developed more often in PPI users (2.23%, 31/1,391) than in PPI nonusers (1.25%, 4,619/370,415), showing a crude RR of 1.787 (95% confidence interval [CI], 1.260 to 2.534; p = 0.002) (Table 2). The results persisted after adjusting for age, sex, presence of osteoporosis, and use of bisphosphonate, calcium, vitamin D, hormone replacement treatment, steroid, and thiazide. In addition, adjusted RRs were higher in PPI users than in H2RA users.
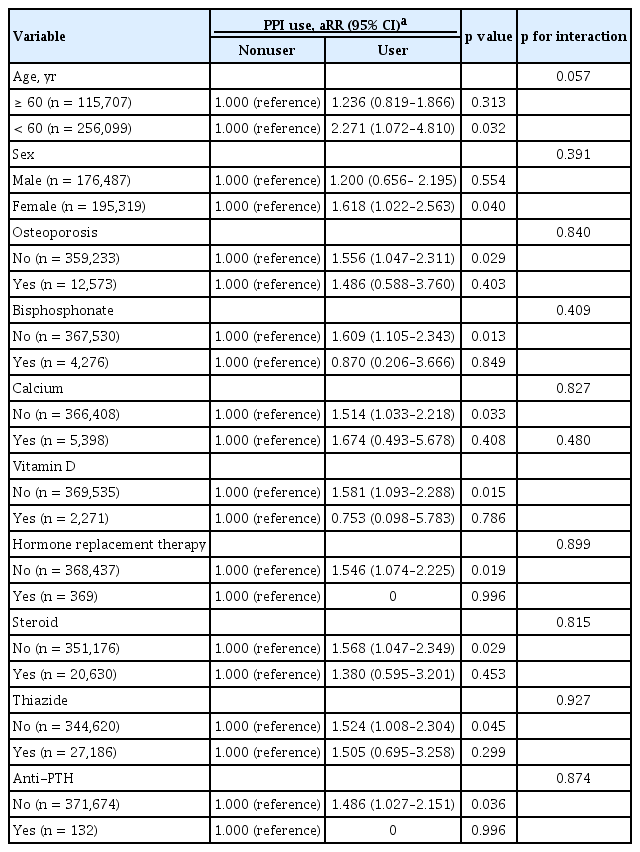
We also evaluated if the association between PPI use and hip fracture development differed in pre-specified subgroups defined by age (≥ 60 and < 60 years), sex (male or female), presence of osteoporosis (no or yes), and use of bisphosphonate (no or yes), calcium (no or yes), vitamin D (no or yes), hormone replacement treatment (no or yes), steroid (no or yes), and thiazide (no or yes) (Table 3). The interaction between the subgroups and PPI effect on fracture were not statistically significant (all p for interaction > 0.05). However, the association between PPI use and hip fracture development was not observed in some subgroups (≥ 60 years, male, osteoporosis, and users of bisphosphonate, calcium, vitamin D, hormone replacement therapy, steroid, thiazide, and anti-PTH).
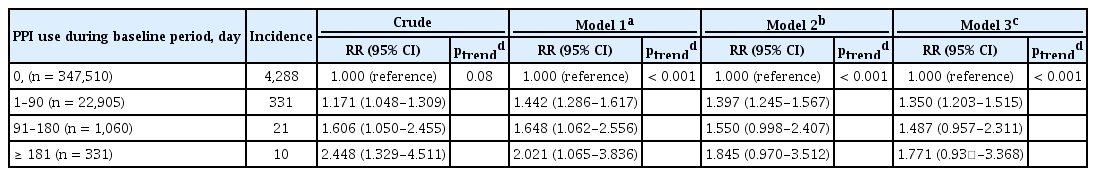
The risk of hip fracture development associated with PPI use increased with duration of PPI use (p trend < 0.001) (Table 4). Compared with PPI never-users (0 day), the fully adjusted RRs of hip fracture development were 1.350 (95% CI, 1.203 to 1.515) for 1- to 90-day users, 1.487 (95% CI, 0.957 to 2.311) for 91- to 180-day users, and 1.771 (95% CI, 0.931 to 3.368) for > 180-day users.
From the 131,689 participants who underwent a health screening, the association between PPI use and hip fracture development was evaluated. Hip fractures developed more often in PPI users (2.29%, 13/568) than in PPI nonusers (0.91%, 1,189/131,121), showing a crude RR of 2.524 (95% CI, 1.470 to 4.333; p = 0.002) (Table 5). The results persisted after adjusting for age, sex, BMI, smoking status, alcohol consumption, physical activity, presence of osteoporosis, and use of bisphosphonate, calcium, vitamin D, hormone replacement treatment, steroid, and thiazide (adjusted RR, 2.025; 95% CI, 1.151 to 3.564; p = 0.014) (Table 5).
DISCUSSION
In this large population-based cohort study, we found that compared to nonusers, PPI users had an increased risk of hip fracture development. The association between PPI use and hip fracture development persisted even after adjusting for factors associated with bone health. Furthermore, our results show that this risk increases with prolonged PPI use, and PPI use increases the risk of hip fracture development more than H2RA use.
Our results are consistent with data from prior studies. In a nested case-control study, Yang et al. [23] reported adjusted odd ratio of 1.44 for hip fracture associated with > 1 year of PPI use. The strength of the association increased with duration of PPI use. Corley et al. [24] a lso demonstrated that patients with hip fractures were more likely to have a previous PPI use of ≥ 2 years than the control. However, the increased risk for PPI use was only present in patients with other fracture risk factors. In a recent large cohort study, Khalili et al. [25] show that chronic PPI use is associated with increased risk of hip fracture even after carefully adjusting for factors. Although the authors insist that the strength of the study is the detailed data on confounding risk factors, there is a possibility of bias in the self-reported survey data. In addition, the study enrolled only postmenopausal women. On the other hand, the present study analyzed definite prescription data from a representative nationwide cohort, and could perform interaction analysis in several clinically relevant subgroups.
Gastric acid suppression induced by PPI use may inhibit calcium absorption, resulting in increasing the risk of hip fracture [31,32].
Recently, a 1-year prospective comparative study showed that PPI use lowers femur neck and total hip BMD [33]. There are, however, conflicting data regarding the effect of PPI use on BMD loss. In two large studies, PPI use was not associated with accelerated BMD loss [37,38]. However, we found that the risk of hip fracture was more modest in H2RA users than in PPI users, and increased with increasing duration of PPI use. These observations indicate that PPI use may increase the risk of hip fracture through its gastric acid suppression effect.
The PPIs are inactive in their native form and are rapidly metabolized by the liver. Thus, maintaining plasma level of the drug is significantly affected by the character of the metabolism, and metabolism of PPIs is dependent on the cytochrome P450 system. CYP2C19 polymorphism is the major component for this [39]. CYP2C19 genotypes were mainly classified into the three groups, and the pharmacokinetics and pharmacodynamics of PPIs differ by CYP2C19 genotypes. Individuals with the poor metabolizer genotype most slowly metabolize among three genotypes, resulting in higher plasma PPI levels than those with the other two genotypes [40]. These CYP2C19 poor metabolic phenotypes are found in 13% to 23% of East Asian populations but in only 2% to 5% of Caucasians [41]. Thus, the effects of PPI, through its gastric acid suppression, on the bone health might be more profound in Koreans than in the Western populations.
In this study, we defined PPI users as those who were prescribed PPIs for more than 90 days during the baseline period because we expected that they may take PPIs at least 1 month every year. Indeed, the annual mean days of PPI prescription during the baseline and follow-up period of 12 years were 40.6 days in PPI user and 3.8 days in PPI non-user (data not shown). This indicates that our PPI user group is consisted of chronic PPI users. Nevertheless, there is a possibility that some subjects changed from PPI user to non-user and vice versa after baseline period. Thus, our results must be interpreted with this limitation in mind.
The interaction between the all pre-specified subgroups and PPI effect on the hip fracture development was not statistically significant. However, the association between PPI use and hip fracture development was not observed in some subgroups (≥ 60 years, male, osteoporosis, bisphosphonate use, and calcium use). These observations indicate that PPI use is not associated with hip fracture in subgroups of ≥ 60 years and male. Indeed, these subgroups (≥ 60 years or male) showed lower RR than < 60 years or female (1.236 vs. 2.271 and 1.200 vs. 1.618, respectively). On the other hand, subgroup without osteoporosis did not show significant association with hip fracture but its RR was similar to that of subgroup with osteoporosis. Moreover, the prevalence of osteoporosis did not differ between PPI users and nonusers at the time of fracture development (PPI user vs. nonuser, 15.71% vs. 25.80%, p = 0.198). Thus, we thought the relatively small size of subgroup with osteoporosis resulted in no statistical significance. In addition, users of bisphosphonate, calcium, vitamin D, hormone replacement therapy, steroid, thiazide, and anti-PTH were small to demonstrate PPI effect on the fracture development.
The present study had some limitations due to its retrospective observational study design. Thus, we tried to minimized potential confounding by adjusting possible confounding factors including many drugs relevant to osteoporosis or influential in bone health. The association between PPI use and hip fracture development also was confirmed in relevant subgroups. In addition, important potential confounders such as BMI, smoking status, alcohol consumption, and physical activity were adjusted in a subgroup analysis. Second, the participants were all Koreans and our findings may not be applicable to other populations. However, this study population, selected by systemic stratified random sampling from the general population, could minimize the possible selection bias. Our study also has several strengths including the long-term longitudinal design that can assess causal relationship and a large sample size. Because PPIs are not over-the-counter drugs in Korea, exact data regarding PPI use is available from the cohort database.
In conclusion, the risk of hip fracture development is higher in PPI users than in nonusers. This association persisted after adjustment for possible confounding factors and grew stronger with increasing duration of PPI use. Thus, our findings suggest that PPI use is associated with hip fracture development.
KEY MESSAGE
1. This large population-based cohort study showed that compared to nonusers, proton pump inhibitor (PPI) users had an increased risk of hip fracture, and the risk increased with prolonged use.
2. This data collectively provides strong support to the hypothesis that PPI use increases the risk of hip fracture through its gastric acid suppression effect. Thus, the need for long-term PPI use should be evaluated with caution among all individuals.
Notes
No potential conflicts of interest relevant to this article are reported.
Acknowledgements
We are indebted to the Big Data Research Study Group under the Korean Society of Neurogastroenterology and Motility for the initiation and support of this study.