Full-dose sofosbuvir plus low-dose ribavirin for hepatitis C virus genotype 2-infected patients on hemodialysis
Article information
Abstract
Background/Aims
New direct-acting antivirals have shown surprising success in the treatment of hepatitis C, not only in the general population, but also in difficult-to-treat cohorts. However, there is still limited data regarding direct-acting antivirals, including sofosbuvir (SOF), in the context of hemodialysis. The aim of this study was to investigate the safety and outcome of administering full-dose SOF (400 mg/day) plus low-dose ribavirin (RBV, 100 to 200 mg/day) in hemodialysis patients with hepatitis C virus (HCV) genotype 2 (GT2) infection.
Methods
Patients with chronic HCV GT2 infection and end-stage renal disease on maintenance hemodialysis treated with full-dose SOF plus low-dose RBV were retrospectively identified from a database of patients with HCV GT2 who were treated in Konkuk University Chungju Hospital between February 2017 and February 2018. Medical records were reviewed for demographics, medical history, laboratory data, and radiologic and electrocardiographic findings.
Results
All nine patients completed a full course of 12 weeks of treatment with a full-dose SOF plus low-dose RBV regimen. Two had compensated cirrhosis. Seven patients were treatment-naïve, and two had a relapse following previous interferon-based therapy. All patients had a sustained viral response at 12 weeks post-treatment. There was no discontinuation of treatment because of side effects.
Conclusions
In hemodialysis patients with HCV GT2 infection, the full-dose SOF plus low-dose RBV regimen appears to be safe and well tolerated, and yields high rates of sustained virologic response.
INTRODUCTION
Globally, there are approximately 71 million people who are chronically infected [1,2]. Furthermore, the prevalence of hepatitis C virus (HCV) infection in hemodialysis (HD) patients is higher than in the general population [3]. The prevalence of anti-HCV-positivity in patients who are on long-term dialysis is below 5% in northern Europe; approximately 10% in most of southern Europe and the United States; and between 10% and 70% in many of the developing countries, including north Africa, Asia, and southern America [4]. According to the report of the Korean Society of Nephrology in 2016, the hepatitis C antibody positivity rate was 4% and was correlated with the duration of HD [5].
HCV is both a cause and a consequence of renal impairment [3]. HCV infection has been also associated with both liver disease-related deaths and cardiovascular mortality in HD patients [6].
New direct-acting antivirals (DAAs), glecaprevir/pibrentasvir (GLE/PIB), offer dramatically improved efficacy not only in the general population but also in difficult-to-treat cohorts, including HD patients. According to recently revised major guidelines of treatment in HCV-infected patients with HD, one interferon-free DAA regimen, GLE/PIB combination therapy, has been approved for HCV genotype 2 (GT2) in patients with HD [7]. GLE/PIB therapy has also been approved and covered for payment under medical care in South Korea since June 2018. The recent Korean Association for the Study of the Liver (KASL) guidelines (November 2017) recommends the combination of GLE/PIB or the combination of peginterferon and low-dose ribavirin (RBV) as current treatment modalities for HCV GT2 patients with severe renal problem (estimated glomerular filtration rate [eGFR] < 30 mL/min/1.73 m2) [8].
Before the recent approval of GLE/PIB, sofosbuvir (SOF) plus RBV was the only regimen covered for payment under the medical care benefits for HCV GT2 patients in South Korea. Management of HCV infection in the Asia-Pacific region is also challenging because of the disparate epidemiology, poor access to all-oral therapy because of availability, cost, or regulatory licensing [9]. However, there are still limited data on the pharmacokinetics, safety, efficacy, and dosage of DAAs, including SOF, in the context of HD [10]. In addition, there is still insufficient clinical data on SOF-based regimens for HCV GT2-infected patients on HD.
The aim of this study was to investigate the safety and outcome of full-dose SOF (400 mg/day) plus low-dose RBV (100 to 200 mg/day) for HCV GT2 infection in HD patients.
METHODS
We retrospectively reviewed the medical records of HD patients with HCV GT2 infection treated with a full-dose SOF (400 mg/day) plus low-dose RBV (100 to 200 mg/day) regimen between February 2017 and February 2018 in Konkuk University Chungju Hospital, Republic of Korea. The study was approved by the Konkuk University Chungju Hospital Institutional Review Board (KUCH 2018-02-003) and conducted in accordance with the ethical guidelines of the Declaration of Helsinki. The written or oral informed consent was waived because of the retrospective study design.
All patients were initiated on HCV non-structural protein 5B (NS5B) inhibitor SOF and antiviral agent RBV combination therapy and were followed up for 12 weeks post treatment. Patients were included if they were aged > 18 years, had chronic GT2 hepatitis C infection, and were undergoing HD. Patients were excluded if they had: (1) decompensated liver cirrhosis; (2) poorly controlled cardiac disease; or (3) any other liver disease including co-infection with hepatitis B virus, autoimmune hepatitis, or primary biliary cholangitis.
At baseline and at 4, 8, and 12 weeks after initiation of treatment, the patients were assessed by physical examination and blood tests. HCV ribonucleic acid (RNA) was estimated by quantitative real-time polymerase chain reaction assay using the COBAS Ampliprep/Cobas Taqman HCV test v.2.0 (Roche Diagnostics GmbH, Mannheim, Germany).
The clinical diagnosis of cirrhosis was based on imaging findings (abdominal sonography and abdominal computed tomography) and compatible clinical features (esophageal varices or thrombocytopenia). The treatment outcome was evaluated based on the sustained virologic response (SVR) rate, which was defined as undetectable serum HCV-RNA (lower limit of detection ≤15 IU/mL) at 12 weeks (SVR12) at the end of treatment [7].
Safety analysis included adverse events (AEs), observed by the patients or their family members and by laboratory findings. AEs were evaluated using the Common Terminology Criteria for AEs version 5.0. AEs were monitored and managed every week during treatment and at 12 weeks after the completion of treatment. Hemoglobin levels were checked every week and, if needed, RBV dose adjustment was carried out based on an AE. We used the following criteria for RBV dose reduction: (1) hemoglobin level < 10 g/dL: reduce RBV dose from 200 to 100 mg/day; (2) hemoglobin level < 8.5 g/dL: discontinue RBV; and (3) neutrophil count < 500/mm3 or platelet count < 50,000/mm3: discontinue RBV. All data regarding histories of cardiovascular disorders and concomitant medications were also reviewed at baseline.
RESULTS
Baseline characteristics of patients
Nine patients with end-stage renal disease on maintenance HD were treated with full-dose SOF (400 mg daily) and low-dose RBV (100 mg once or twice a day) for a total of 12 weeks. Seven patients (78%) were naïve to treatment and two (22%) were treatment experienced, all of them with pegylated interferon and RBV (two with relapse). Two patients had Child-Pugh class A cirrhosis. Baseline characteristics are summarized in Table 1.
Efficacy
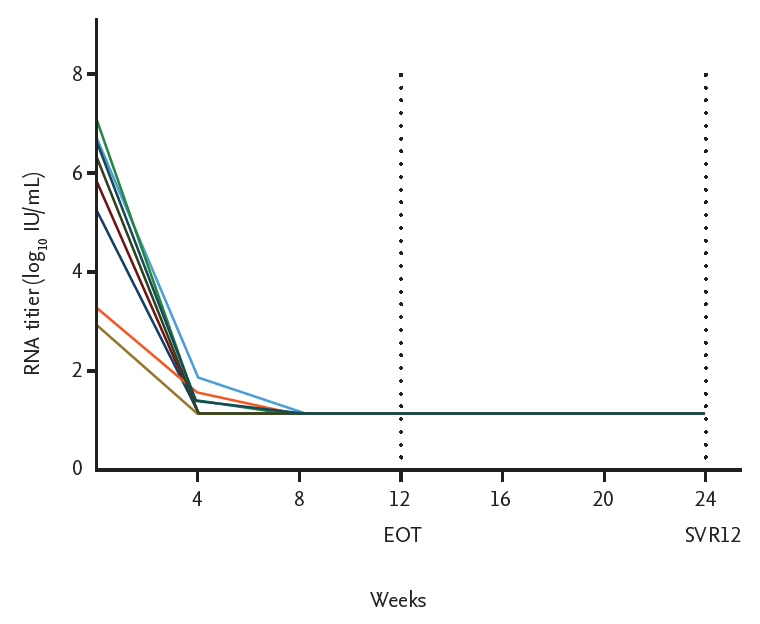
All patients completed 12 weeks of treatment with full-dose SOF (400 mg daily) plus low-dose RBV (100 to 200 mg daily) and were followed up for 12 weeks after the end of treatment. SOF dose adjustment was not made during the treatment period. RBV dose adjustments were made during the treatment period based on clinical symptoms or laboratory findings. Six patients (66.6%) were aviremic by week 4 and all patients were aviremic by week 8 and remained so until the end of treatment. SVR12 was achieved in all patients (Fig. 1).
Safety
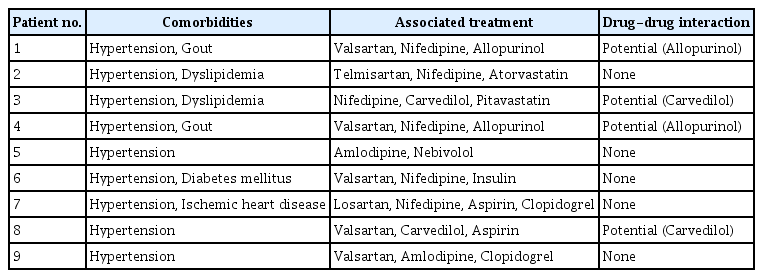
All patients in this study were on maintenance HD. Before the initiation of antiviral therapy, we reviewed the comedications and checked whether there were drugdrug interactions between the comedications and the antiviral agents, SOF and RBV (https://www.hep-druginteractions.org) (Table 2). Potential drug-drug interactions were observed in four patients (44.4%): between RBV and carvedilol in two patients and between RBV and allopurinol in two patients.
All patients were received regimens containing RBV, with five patients (5 5.5%) developing worsening anemia (Table 3). The starting doses of RBV were variable: 200 mg daily in six patients and 100 mg daily in three patients. Hemoglobin levels were monitored every week in all patients. For patients with worsening anemia, the dose of RBV was reduced in four patients and had to be temporarily discontinued in one patient until the base-line hemoglobin level was reached. Anemia resolved with dose reduction of RBV and erythropoietin-stimulating agents. There was no serious AE in any of the patients in the present study.
DISCUSSION
This study demonstrated that treatment with full-dose SOF plus low-dose RBV for 12 weeks has excellent efficacy and safety for HD patients with HCV GT2 infection.
SOF is a nucleotide-analogue prodrug inhibitor of the NS5B RNA-dependent polymerase, an essential protein for replication of HCV [11]. This drug is phosphorylated by the liver into the active metabolite GS-461203. Dephosphorylation of GS-461203 results in the formation of the inactive metabolite GS-331007 [12]. Approximately 78% of the inactive metabolite is eliminated renally. After 400-mg SOF was administered in patients with severe renal impairment, the area under the curves of SOF and GS-331007 were increased by 171% and 451%, respectively [13]. Thus, currently, SOF dose recommendations cannot be given to patients with severe renal impairment (eGFR < 30 mL/min/1.73 m2) or with end-stage renal disease because of higher exposures (up to 20-fold) to GS-331007 [7].
In the recently revised major treatment guidelines or guidance, the treatment modality for HCV GT2 patients with severe renal impairment or end-stage renal disease on HD is as follows: (1) the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (2017) recommend that GT1–6 HCV-infected patients with chronic kidney disease (CKD) stage 4 or 5 (eGFR <30 mL/min/1.73 m2 or end-stage renal disease) should be treated with 8 to 16 weeks of GLE/PIB [14]; (2) the general recommendations of the European Association for the Study of the Liver (EASL) Recommendations on Treatment of Hepatitis C (2018) state that patients infected with all GTs and with severe renal impairment (eGFR < 30 mL/min/1.73 m2) or with endstage renal disease on HD should be treated with 8 to 12 weeks of GLE/PIB [7]; (3) in addition to these, according to the EASL guidelines, SOF should be used with caution in patients with an eGFR < 30 mL/min/1.73 m2 or with end-stage renal disease, only if an alternative treatment is not available, because dose recommendations cannot currently be given for these patients [7].
Although DAA-based treatment regimens without SOF and RBV may be a new trend to treat patients with severe renal impairment, there is accumulating evidence on the safe use of SOF-based regimens in patients with an eGFR of < 30 mL/min/1.73 m2, including patients on HD (Table 4) [7,15]. Moreover, if treatment is urgent and no SOF-free regimen is available, the risk versus the benefit of using SOF-based regimens should be carefully weighed.

Summary of studies related to sofosbuvir-based regimens in patients with severe renal impairment including cohorts with HCV GT2-infected patients
Gane et al. [16] presented the results for 10 HCV GT1-infected patients with severe renal impairment. They were treated with low-dose SOF (200 mg/day) plus low-dose RBV (200 mg/day). This low-dose SOF-based regimen showed low efficacy. Desnoyer et al. [15] reported that SOF and its active metabolite did not accumulate in patients on HD and suggested administering full-dose SOF (400 mg/day) with close monitoring. In our study, all patients were treated with full-dose SOF-based regimen for 12 weeks and they demonstrated excellent SVR12. The previously reported data and our data suggested that the dose of SOF could be an important variable to reach SVR in HD patients and that a reduced dose might lead to sub-therapeutic exposure and the risk of treatment failure [15,17]. Therefore, full-dose SOF regimen might be more effective to reach SVR in HD patients.
Saxena et al. [18] also reported the real-world outcomes of full-dose SOF-based therapy in patients with renal impairment. Among those CKD patients, five were treated with HD, and all of them achieved SVR12. Cox-North et al. [19] suggested that full-dose SOF-based antiviral therapy ± RBV yielded 97% SVR12 in patients with severe renal impairment. Recently, Agarwal et al. [20] reported on the safety and efficacy of full-dose SOF-based therapy in 62 patients on maintenance HD. Only one patient had HCV GT2 infection in this study [20]. The previous studies listed above showed that full-dose SOF-based therapy achieved an SVR12 close to 90% in HCV patients on HD (Table 4). The most common GT was GT1 in most of the studies. There is insufficient clinical data on full-dose SOF plus low-dose RBV in GT2 patients on maintenance HD. In the present study, all the patients had GT2 and all of them achieved SVR12. This finding is compatible with the previous results in GT1 HCV-infected patients on HD treated with full-dose SOF-based therapy. This result is also compatible with the previously reported data in the general population with HCV GT2 infection.
In the present study, the safety during full-dose SOF treatment was evaluated at baseline and at 4, 8, and 12 weeks after initiation of treatment by physical examination and laboratory tests including a full blood count, renal profile, and liver function profile. In addition, the safety during low-dose RBV treatment was monitored every week during treatment by laboratory tests including a full blood count. Seven patients had complications during antiviral therapy: five had aggravation of anemia, one patient had itching, and one had fatigue. Although anemia worsened in over half of the patients, no patient discontinued the treatment because of complications. In previously available data and our data, the main AE was anemia related to the use of RBV. The anemia resolved with dose reduction of RBV and erythropoietin-stimulating agents. The previously reported data and our data also showed that many HD patients demonstrated good tolerance to this antiviral regimen with close monitoring during the therapy.
Since patients on HD took different types of comedications due to their comorbidities, drug-drug interactions seemed to make it difficult for them to receive antiviral agents. Although four patients had potential drug-drug interactions between their comedications and the antiviral agents, all of them completed antiviral treatment without early discontinuation in our study. There were no serious AEs related to potential drugdrug interaction.
Although the present study showed high rates of SVRs in HD patients with HCV GT2 infection, recently revised KASL guidelines recommends the combination of GLE/PIB for the treatment of HCV infection in patients with HD, and this makes it difficult to use the full-dose SOF and low-dose RBV regimen as a therapeutic option for the treatment of hemodialytic patients in South Korea because of potential of AEs related to this regimen. However, GLE/PIB treatment is still challenging in Asian-Pacific countries because of the poor access to GLE/PIB. Therefore, the full-dose SOF and low-dose RBV may be an alternative treatment option in those countries, such as India and China, that have an impediment for the access to GLE/PIB treatment for HCV GT2 infection in HD patients.
The present study has several limitations. Firstly, this study had a retrospective study design with a small number of patients. Further studies with larger sample sizes and modified designs are needed to verify the findings suggested in this study. Secondly, the drug levels of SOF and its metabolites were not evaluated in this study. The optimal dosing of SOF needs to be studied with careful measurements of drug and metabolite levels in order to provide clinicians with helpful information for an effective and tolerable treatment option. However, our clinical data suggests that the full-dose SOF plus low-dose RBV regimen may be tolerated and effective in the treatment of HCV GT2 infection in HD patients and could be another therapeutic option for HCV GT2-infected patients on HD in countries where the recommended drugs are not available.
In conclusion, the full-dose SOF (400 mg once daily) plus low-dose RBV (100 mg once or twice daily) regimen appears to be safe and well tolerated, and yields rapid virologic suppression and high rates of SVRs in HD patients with HCV GT2 infection. Further studies with larger cohorts are needed to verify the efficacy and safety of this regimen in HD patients.
KEY MESSAGE
1. The full-dose sofosbuvir (SOF) plus low-dose ribavirin (RBV) regimen appears to be safe and well tolerated, and yields high rates of sustained virologic responses in hemodialysis (HD) patients with hepatitis C virus (HCV) genotype 2 (GT2) infection.
2. The full-dose SOF plus low-dose RBV regimen could be another therapeutic option for HCV GT2-infected patients on HD in countries where the recommended drugs are not available.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This paper was written as part of Konkuk University’s research support program for its faculty on sabbatical leave in 2018.