Supplementary nutrients for prevention of vascular calcification in patients with chronic kidney disease
Article information
Abstract
Vascular calcification (VC) and malnutrition associated with cardiovascular disease are common in patients with chronic kidney disease (CKD) treated with dialysis. VC, which reflects vascular aging, and malnutrition are also encountered in the non-CKD elderly population. This similarity of clinical findings suggests that the progression of CKD is related to aging and the existence of a causal relationship between VC and malnutrition. To retard renal progression, a low- or very-low-protein diet is usually recommended for CKD patients. Dietary education may induce malnutrition and deficiency of important nutrients, such as vitamins K and D. Menaquinone-7, a type of vitamin K2, is under investigation for inhibiting VC in elderly patients without CKD, as well as for prevention of VC in patients with CKD. Nutritional vitamin D, such as cholecalciferol, may be considered to decrease the required dose of active vitamin D, which increases the risk of VC due to increased calcium and phosphate loads. Omega-3 fatty acids are important nutrients and their ability to inhibit VC needs to be evaluated in clinical trials. This review focuses on the ability of supplementary nutrients to prevent VC in patients with CKD, in whom dietary restriction is essential.
INTRODUCTION
Patients with chronic kidney disease (CKD) are at greater risk of cardiovascular disease (CVD), as compared to the general population. The mortality rate of a 30-year-old patient with CKD on dialysis is similar to that of an 80-year-old person without CKD [1,2]. Vascular calcification (VC) associated with CVD is commonly found in patients with CKD who are on dialysis (Fig. 1). VC of the aorta and coronary artery, which reflects aging of these vessels, is also encountered in elderly persons without CKD (Fig. 1D). Malnutrition is related to CVD and is frequently found in patients with advanced CKD and even in elderly persons without CKD [3]. These epidemiologic findings imply that CKD progression and VC are related to aging. In addition, there is likely to be a causal relationship between VC and malnutrition, particularly in patients with CKD.
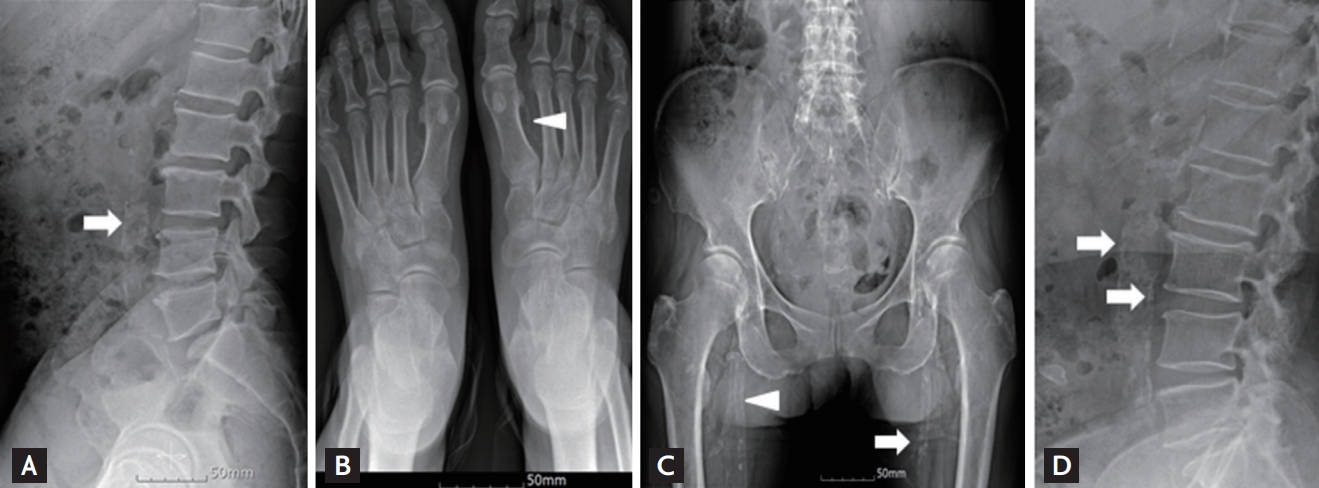
Plain radiographs showing vascular calcification (VC) in patients with chronic kidney disease (CKD) (A-C) and an elderly man without CKD (D). (A) Abdominal aortic calcification (AAC) on a lateral lumbar spine view, (B) arterial medial calcification of the dorsalis pedis artery of the feet, and (C) arterial intimal and medial calcification in the pelvis. (D) AAC in an 86-year-old man without CKD. Arrow, arterial intimal calcification; arrowhead, arterial medial calcification on plain radiographs.
Patients with CKD are reluctant to eat high phosphate- and protein-containing foods because of concern over progression of their renal disease. Patients with CKD treated with dialysis are unable to intake sufficient quantities of nutrients because of anorexia and nausea. Dietary education focused on restricting the intake of harmful dietary components, including phosphorous and potassium, may reduce the dietary intake. Finally, patients with CKD have deficiencies of important nutrients, such as vitamins K and D, and so are at high risk of malnutrition. The authors have reviewed supplementary nutrients for prevention of VC in patients with CKD on a restricted diet and poor dietary intake.
EVALUATION OF VASCULAR CALCIFICATION
The severity of VC can be evaluated by electron beam computed tomography (CT), multi-slice CT, or plain radiographs. Coronary artery calcification (CAC) on CT is associated with coronary plaque accumulation and a poor outcome in patients with CKD [4,5]. VC is easily evaluated using plain radiographs, which are inexpensive and involve less exposure to radiation than CT. Plain radiographs of the lateral lumbar spine, hands and pelvis (HP), feet, and chest, can be used to evaluate and score VC [6-12]. The abdominal aortic calcification (AAC) or HP VC score on plain radiographs is associated with coronary artery disease (CAD) in patients on dialysis [6,13,14]. The presence of VC on plain radiographs of the feet is associated with peripheral artery disease as well as CAD [9,10]. Among the various VC scoring systems, the AAC score on plain radiographs of the lateral lumbar spine is predictive of CAC on CT and the T-score (an indicator of bone mineral density) of the forearm [15,16]. The 2017 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend that VC be evaluated using a lateral view of the lumbar spine [17]. Plain radiographs are also useful for monitoring of VC progression [18]. Patients on dialysis with advanced VC are at increased risk of mortality [19].
VC may manifest as arterial intimal or medial calcification [20,21]. Arterial intimal calcification is found in the coronary arteries, carotid arteries, and aorta, and arterial medial calcification in the peripheral arteries of the hands and feet [22]. Ring-like calcification of the dorsalis pedis artery or linear railroad-track form calcification on plain radiographs indicates arterial medial calcification (Fig. 1). On plain radiographs, arterial intimal calcification appears as areas of irregular and patchy density (Fig. 1).
RISK FACTORS FOR VASCULAR CALCIFICATION
Arterial intimal and medial calcification often coexist in patients with CKD; however, whether these entities share risk factors is unclear. Traditional risk factors, such as aging, diabetes mellitus, hypertension, and dyslipidemia, as well as inflammation, oxidative stress, klotho deficiency, and high calcium and phosphate loads play crucial roles in the pathogenesis of intimal and medial VC [23-25]. VC is prevalent in diabetic patients with CKD and hyperglycemia induces the development and progression of VC [26].
Hyperphosphatemia is a risk factor for death in both the general population and in patients with CKD [27,28], and can worsen VC [29,30]. Egg white has an ideal phosphorus‑to‑protein ratio (1.42 mg/g protein), while red meat, pork, and poultry have low levels of phosphorus (4.1 to 9.2 mg/g protein) [31,32]. However, these foods have several additives that contain inorganic phosphorus, which is readily absorbed in the intestine. The acceptable phosphorus‑to‑protein ratio of food in terms of minimizing the risk of VC is < 16 mg/g, and reducing the use of food additives containing inorganic phosphorus is important.
Use of calcium-free phosphate binders decreases mortality and retards the progression of VC compared to calcium-containing phosphate binders in patients on dialysis [33,34]. The 2017 KDIGO guidelines recommend restriction of the use of calcium-based phosphate binders to reduce the phosphorus level [17]. Differentiation of vascular smooth muscle cells to osteoblast-like cells is involved in the pathogenesis of VC and calcium may precipitate on the osteoblast-like cell. An elevated calcium load due to use of calcium-containing phosphate binders, high-dose active vitamin D analogs, and a high dialysate calcium concentration can accelerate the progression of VC. Use of low dialysate calcium (1.25 mmol/L) may reduce the progression of CAC in patients on hemodialysis (HD) [35,36]. The progression of CAC after use of low dialysate calcium (1.25 mmol/L) compared to standard dialysate calcium (1.5 mmol/L) has been investigated in patients on HD [37]. However, patients in the low-calcium dialysate group received higher doses of calcium-containing phosphate binders or calcitriol. To avoid unnecessary calcium loading, the most recent KDIGO guidelines recommend that mild and asymptomatic hypocalcemia not be treated.
NUTRIENT DEFICIENCY AND VASCULAR CALCIFICATION
Protein restriction and vascular calcification
The KDIGO recommends lowering protein intake to 0.8 g/kg/day in patients with CKD with a glomerular filtration rate of < 30 mL/min/1.73 m2 and limiting protein intake to 1.3 g/kg/day in adults with CKD at risk of progression [38]. Excess dietary protein intake leads to the retention of nitrogenous metabolites and uremic toxins. In contrast, insufficient protein intake can result in loss of lean body mass, deficiencies of essential nutrients, inflammation, and VC in patients with CKD [39,40]. Induction (by feeding 0.75% adenine for 4 weeks) of arterial medial calcification in uremic rats dramatically increased the frequency and severity without significantly affecting the elevated serum creatinine, phosphate, and parathyroid hormone (PTH) levels after changing dietary protein composition from 25% to 2.5% [41]. Therefore, only a very‑low‑protein diet (VLPD) induces VC in the presence of uremia; however, the underlying mechanism needs to be elucidated. Physicians and dieticians encourage patients with CKD to avoid foods containing high phosphate, potassium, salt, and protein levels [42,43]. Intake of green leafy vegetables with high levels of vitamin K increases the risk of hyperkalemia, and intake of cheese with high levels of vitamins K and D increases the serum phosphate level. However, strict education on dietary restriction may induce malnutrition and deficiency of important nutrients such as vitamin K and nutritional vitamin D, which is related to VC.
In a recent randomized control trial, a ketoanalogue-supplemented vegetarian VLPD (0.3 to 0.4 g/kg/day) in patients with high compliance slowed CKD progression compared to a conventional low-protein diet (0.6 g/kg/day) [44,45]. Notably, nutritional vitamin K and vitamin D replacement with pills (such as a ketoanalogue) should be considered to prevent VC when physicians and dieticians recommend VLPD to delay the need for dialysis in patients with CKD.
Vitamin D and vascular calcification
As renal function declines, phosphate retention results in increased levels of fibroblast growth factor-23 (FGF-23) and PTH. In addition, the decreased serum-free calcium level caused by reduced production of calcitriol and increased catabolism of active vitamin D in patients with CKD activates PTH secretion [46]. A normal serum phosphate level is maintained in patients with mild‑to‑moderate CKD because of the elevated serum PTH and FGF-23 levels. The 2009 KDIGO guidelines recommend that secondary hyperparathyroidism (SHPT) in patients with CKD can be controlled using calcitriol and vitamin D analogs [47]. Calcitriol and other 1α-hydroxylated vitamin D sterols, such as 1α-hydroxyvitamin D3 (alfacalcidol), 1α-hydroxyvitamin D2 (doxercalciferol), and 19-nor-1α-25-dihydroxyvitamin D2 (paricalcitol), are commonly prescribed for SHPT. Indeed, oral calcitriol use may be positively associated with survival in patients with CKD [48,49]. However, these drugs not only increase the risk of hypercalcemia but also do not improve the clinical outcomes. Therefore, the 2017 KDIGO guidelines recommend use of calcitriol and vitamin D analogs exclusively in patients with severe and progressive SHPT [17]. Calcimimetics such as cinacalcet are the first‑line therapy to reduce the PTH level. Cinacalcet binds to the transmembrane region of the calcium-sensing receptor. While treatment with vitamin D analogs reduces the PTH level but can lead to the development of VC by increasing the intestinal absorption of calcium and phosphate [50-52], cinacalcet reduces the PTH level and decreases the serum calcium and phosphorus levels [53]. Analogues of calcitriol, such as doxecalciferol, paricalcitol, and maxacalcitol, have less calcemic activity than calcitriol. Maxacalcitol, previously called 22-oxacalcitriol, ameliorates VC to a greater degree than calcitriol despite their comparable ability to control the levels of PTH and calcium-phosphate products [51]. This may be due to the differential effects of vitamin D receptor activators on VC in the presence of uremia. Paricalcitol and doxecalciferol are analogs in the vitamin D2 family; however, paricalcitol induces a greater reduction in calcium and phosphate levels and amelioration of VC than doxecalciferol [54,55]. Combination treatment with cinacalcet and a low dose of active vitamin D reportedly decreases the Agatston CAC score and the volume CAC score compared to flexible higher-dose active vitamin D monotherapy in HD patients [56]. This suggests that higher-dose active vitamin D monotherapy without cinacalcet influences the calcification score by increasing the calcium and phosphate loads.
Use of nutritional vitamin D, such as ergocalciferol and cholecalciferol, can decrease the required dose of active vitamin D (Fig. 2). Supplementation of nutritional vitamin D reportedly improves the vitamin D status and decreases the PTH level in patients with CKD [57-60]. In particular, cholecalciferol supplementation in patients on dialysis allowed the dose of active vitamin D to be reduced and did not increase the calcium or phosphate level [61]. In a recent study, treatment with cinacalcet, calcitriol, and cholecalciferol accelerated achievement of the target PTH (≤ 300 pg/mL), reduced the mean dose of calcitriol, increased the 25-hydroxyvitamin D level, and increased the femur-neck bone density compared to cinacalcet, calcitrol, and placebo [62]. The changes in calcium and phosphorus levels were similar in the two treatment arms. Reduced use of active vitamin D may prevent VC. Therefore, nutritional vitamin D can be considered an alternative to active vitamin D. Vitamin D therapy should be carefully prescribed, and strict control of the patient’s calcium and phosphate intake is necessary. Further studies are needed to determine whether nutritional vitamin D supplementation slows the development or progression of VC.

Mechanism by which vitamin D modulates arterial intimal and medial calcification in patients with chronic kidney disease. Bold arrow indicates more related with arterial intimal vascular calcification than thin arrow. Bold circle nutritional vitamin D for prevention of vascular calcification in patients with chronic kidney disease. PTH, parathyroid hormone.
Vitamin K and vascular calcification
The vitamin K group is composed of phylloquinone (vitamin K1), several menaquinones (MK, vitamin K2), and synthetic forms, such as menadion (vitamin K3) and esterified menadion (vitamin K4). The main sources of dietary vitamin K1 are green leafy vegetables and some plant oils, while dietary vitamin K2 is abundant in fermented foods, such as cheese and Japanese natto [63,64]. Phylloquinone is converted into MK-4. The characteristics of MKs are dependent on the number of isoprenoid residues in the side chain. For example, MK-7 has a half-life of 3 days and MK-4 of 1 hour. Among the several isoforms of MK-n and long-chain MKs, human foods are particularly rich in MK-7.
Although the data are controversial, accumulating evidence suggests that a low vitamin K level is associated with osteoporosis and increased risk of fracture, CVD, and mortality [65-67]. A high dietary vitamin K intake may benefit bone and vascular health. Indeed, a relationship between diet-induced vitamin K deficiency and VC has been reported [67,68]. In patients on dialysis, a low vitamin K intake may be related to VC [69,70]. Warfarin, a vitamin K antagonist, can induce calciphylaxis, also known as calcific uremic arteriopathy, which is a life-threatening, rare complication of end-stage renal disease [71,72]. In this respect, dietary vitamin K supplementation has a beneficial effect on VC [73]. In mammals, vitamin K serves as a cofactor for γ-glutamyl carboxylation, which converts glutamate into γ‑carboxyglutamate (Gla). Under vitamin K insufficiency, undercarboxylated Gla-proteins, such as uncarboxylated osteocalcin (OC) and desphosphorylated-uncarboxylated matrix Gla-protin (MGP) are released in the circulation because of reduced carboxylation. The circulating levels of undercarboxylated Gla-proteins are associated with poor bone and vascular health [74-77]. VC is positively associated with the levels of undercarboxylated Gla-proteins and medial arterial calcification occurs in areas of deposition of undercarboxylated Gla-proteins. In contrast, increased vitamin K intake can increase carboxylation of OC and MGP (Fig. 3) [78-81]. Moreover, MK-7 may be the optimal form for dietary supplementation because of its long half-life. MK-7 supplementation decreased the desphosphorylated-uncarboxylated MGP levels in patients on chronic HD [73]. MK-7 (360 μg) is also under investigation for prevention of VC in patients with a baseline Agatston CAC score of 50 to 400 [82]. Therefore, MK-7 shows promise for the prevention of VC in patients with CKD. However, studies of the efficacy and safety of vitamin K supplementation in patients with CKD are lacking and so further prospective research is needed.

Mechanism by which vitamin K modulates arterial intimal and medial calcification in patients with chronic kidney disease. Bold arrow indicates more related with arterial medial vascular calcification than thin arrow. Bold circle, vitamin K for prevention of vascular calcification in patients with chronic kidney disease. MGP, matrix γ-carboxyglutamate protein.
Fatty acids and vascular calcification
Among the risk factors for CVD, dyslipidemia plays a crucial role in CAC [83,84]. Atherosclerotic lesions with cholesterol and lipid deposition are also associated with VC [85,86]. Omega-3 fatty acids (FAs) was used for the treatment of a lipid abnormality characterized by hypertriglyceridemia in patients with CKD [83,87] and it decreased aortic calcification and warfarin induced medial arterial calcification in a rat model [88,89]. The content of oleic acid, a monounsaturated fatty acid (MUFA), in the erythrocyte membrane was significantly higher in patients with acute coronary syndrome and those on dialysis [90-93]. MUFA is endogenously synthesized from dietary carbohydrates and saturated FA intakes, and this process can be aggravated in the presence of uremia [94,95]. Omega-3 FAs, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), decrease the oleic‑acid level in patients on dialysis [96,97]. Moreover, the erythrocyte membrane contents of MUFAs, including oleic acid, were significantly higher in patients with arterial medial calcification of the feet than in those without calcifications [93]. Therefore, omega-3 FAs not only control the erythrocyte membrane FA content but also ameliorate VC.
VC is associated with arterial stiffness and an increased CV risk [98], while an EPA- or DHA-rich diet ameliorates arterial stiffness [99,100]. Fetuin-A, which is synthesized in the liver, is a circulating inhibitor of VC [101]. In patients on dialysis, a low fetuin-A level is associated with malnutrition, arterial stiffness, VC, and mortality [102-104]. We reported previously that omega-3 FA supplementation increases the fetuin-A and 1, 25 dihydroxyvitamin D levels in patients on dialysis [96], suggesting that omega-3 FA supplementation activates vitamin D. Co-supplementation of omega-3 FA and cholecalciferol may have a clinical benefit by activating vitamin D, increasing the fetuin-A level, modifying the erythrocyte membrane FA content, and reducing use of active vitamin D (Fig. 4). Further clinical trials to identify the role of omega-3 FA in VC are needed.

Mechanism by which omega-3 fatty acids modulate arterial intimal and medial calcification in patients with chronic kidney disease. Supplementation of several nutrients may be more useful for prevention of vascular calcification.
This review was limited by being based on mainly experimental studies; few clinical studies of VC prevention using supplementary nutrients in patients with CKD were analyzed.
CONCLUSIONS
Nutritional management, such as a VLPD, may be necessary to delay the progression of renal disease; however, deficiencies in essential nutrients, such vitamins K2 and D, must be overcome to prevent VC. Cholecalciferol, MK, and omega-3 FA are promising supplementary nutrients for preventing VC in patients with CKD. Further studies are needed to clarify the ability of supplementary nutrients to prevent intimal or medial VC in patients with CKD.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the Dong-A University research fund.
