Clinical activity of fulvestrant in metastatic breast cancer previously treated with endocrine therapy and/or chemotherapy
Article information
Abstract
Background/Aims
We conducted a retrospective analysis of the clinical activity of fulvestrant in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer (MBC) previously treated with endocrine therapy and/or chemotherapy.
Methods
We reviewed the medical records of all patients with MBC treated at Samsung Medical Center between January 2009 and August 2016. Patients received fulvestrant 250 mg intramuscularly every 28 days (from January 2009 to November 2010) or 500 mg intramuscularly every 28 days (from December 2010 to August 2016). Tumor responses were assessed every 8 weeks and at the end of treatment, as well as when disease progression was suspected.
Results
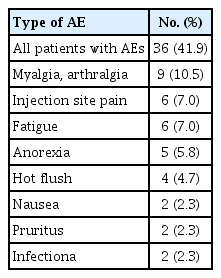
A total of 84 patients were included in this study. A median of two previous endocrine treatments had been performed; 79% of the patients had received two or more endocrine treatments. Forty-five patients (54%) had been treated with chemotherapy for MBC before the fulvestrant treatment course. Visceral metastasis was found in 49 patients (58%). The estimated median progression-free survival and overall survival were 4.4 months (95% confidence interval [CI], 3.4 to 5.5) and 32.5 months (95% CI, 17.6 to 47.4), respectively. The disease control rate was 40.5% (95% CI, 30.5 to 51.5); partial response was observed in 16% of the patients and stable disease was observed in 25% of the patients. The most frequently reported adverse reactions were mild-to-moderate grade myalgia (10.5% of the patients), injection site pain (7%), and fatigue (7%). Fulvestrant was generally well tolerated.
Conclusions
Fulvestrant showed encouraging clinical activity and favorable feasibility in postmenopausal women with MBC who had been treated with multiple endocrine therapies and/or cytotoxic chemotherapies.
INTRODUCTION
Aromatase inhibitors (AIs) and tamoxifen are standard therapeutic options in the first-line treatment of postmenopausal patients with hormone receptor-positive metastatic breast cancer (MBC) [1]. Despite receiving appropriate treatment, the majority of patients eventually progress or relapse during or after first-line endocrine therapy. These patients are often treated with sequential endocrine therapy. Fulvestrant is a selective estrogen receptor (ER) down-regulator that competitively binds to ERs and induces a conformational change [2,3]. It does not show cross-resistance with tamoxifen or the ER agonist activity associated with tamoxifen [3]. The clinical efficacy of fulvestrant has been demonstrated in patients with ER-positive breast cancer that was previously untreated or treated with endocrine therapy [4-7].
Recently, several novel agents were developed to overcome resistance to endocrine therapies. Palbociclib is an orally bioavailable selective inhibitor of cyclin-dependent kinase 4 and 6 (CDK4 and CDK6) that prevents DNA synthesis by blocking progression of the cell cycle from G1 to S phase [8,9]. On February 19, 2016, the U.S. Food and Drug Administration approved palbociclib for use in combination with fulvestrant for the treatment of women with ER-positive, human epidermal growth factor receptor 2 (HER2)-negative MBC with disease progression following endocrine therapy [10]. In the PALOMA-3 (Palbociclib Ongoing Trials in the Management of Breast Cancer 3) study, fulvestrant plus palbociclib was compared with fulvestrant plus placebo in patients with MBC that progressed on previous endocrine therapy; the median follow-up was 8.9 months. The median progression-free survival (PFS) was 9.5 months (95% confidence interval [CI], 9.2 to 11.0) in the fulvestrant plus palbociclib group and 4.6 months (range, 3.5 to 5.6) in the fulvestrant plus placebo group (hazard ratio [HR], 0.46; 95% CI, 0.36 to 0.59; p < 0.0001) [11]. Fulvestrant thus became the preferred regimen partner of the CDK4 and CDK6 inhibitor palbociclib. In this context, here we focused on the specific clinical role of fulvestrant.
The results of a clinical trial comparing fulvestrant 250 mg with anastrozole in postmenopausal women with MBC who showed disease progression after receiving endocrine treatment have been reported. The median time to progression (TTP) was 5.5 months in the fulvestrant arm and 4.1 months in the anastrozole arm; the overall response rate (ORR) was 19.2% and 16.5% for fulvestrant and anastrozole, respectively (95% CI, 2.27 to 9.05; p = 0.31) [6]. The multicenter phase III Evaluation of Faslodex versus Exemestane Clinical Trial (EFECT) trial demonstrated similar efficacy of fulvestrant at 250 mg compared with exemestane at 25 mg orally once-daily. The median TTP was 3.7 months in both groups (p = 0.653) and the ORR was 7.4% and 6.7% in the fulvestrant arm and the exemestane arm (p = 0.736), respectively [7].
However, few studies have reported data from Korean postmenopausal patients with MBC who were treated with fulvestrant. Moreover, objective data on fulvestrant are scarce. Knowledge of the clinical activity of fulvestrant would be informative to clinicians and helpful for patients. Here we report the clinical activity of fulvestrant for the treatment of postmenopausal women with hormone receptor-positive, HER2-negative MBC previously treated with endocrine therapy and/or chemotherapy.
METHODS
Patients
We retrospectively reviewed the medical records of all patients with MBC treated at Samsung Medical Center between January 2009 and August 2016. Eligible patients were postmenopausal women with MBC who had been treated with fulvestrant and whose tumors were ER-positive and/or progesterone receptor-positive. Patients with HER2-positive breast cancer and those who had received fulvestrant in clinical trials were excluded. Patients received fulvestrant 250 mg intramuscularly every 28 days (from January 2009 to November 2010) or 500 mg intramuscularly every 28 days (from December 2010). We collected clinical data including baseline patient and tumor characteristics, prior endocrine and cytotoxic chemotherapies, and radiological findings. All procedures involving patients were reviewed and approved by the Institutional Review Board (IRB) of Samsung Medical Center, which waived informed consent (No. 2016-12-092).
Response evaluation
According to the relevant guidelines and our department policies, tumor response was assessed every 8 weeks (± 1 week) and at the end of treatment, as well as when disease progression was suspected. Tumor responses were assessed by appropriate imaging techniques such as computed tomography, bone scans, and magnetic resonance imaging if indicated. The primary endpoint of this study was PFS; the secondary endpoints included overall survival (OS), disease control rate (DCR), and safety. The DCR included the rates of complete response, partial response (PR), and stable disease (SD). The responses were classified according to the Response Evaluation Criteria in Solid Tumors version 1.1 [12]. Adverse events were collected and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Statistics
Descriptive statistics are reported as proportions and medians. Kaplan-Meier estimates were used for the analysis of all time-to-event variables and the 95% CI for the median time to event was computed. OS was measured from the date of fulvestrant treatment to the date of death from any cause and was censored at the date of the last follow-up visit. PFS was calculated from the date of fulvestrant treatment to the date of disease progression, death from any cause, or the last follow-up. The DCR is presented with a 95% CI. Univariate and multivariate analyses examined the impact of clinical and treatment parameters on the survival outcome using Cox proportional hazards analyses. Variables used to identify prognostic parameters for PFS and OS were age, presence of visceral disease, stage at diagnosis, prior adjuvant endocrine therapy, prior cytotoxic therapy, time to surgery to recurrence, and time from first-line endocrine therapy to fulvestrant. Nonsignificant variables were dropped individually, beginning with the least significant variable. Variables with p < 0.05 were considered significant for the analysis, and the 95% CIs were calculated. All statistical analyses were performed using PASW Statistics version 23.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
Between January 2009 and August 2016, a total of 200 patients with MBC were treated with fulvestrant. Among them, 116 patients were excluded because they were taking part in a clinical study, had HER2-positive MBC, or lacked available follow-up medical records. Thus, a total of 84 patients were finally included in this study. Fig. 1 shows a flow chart of patient inclusion.
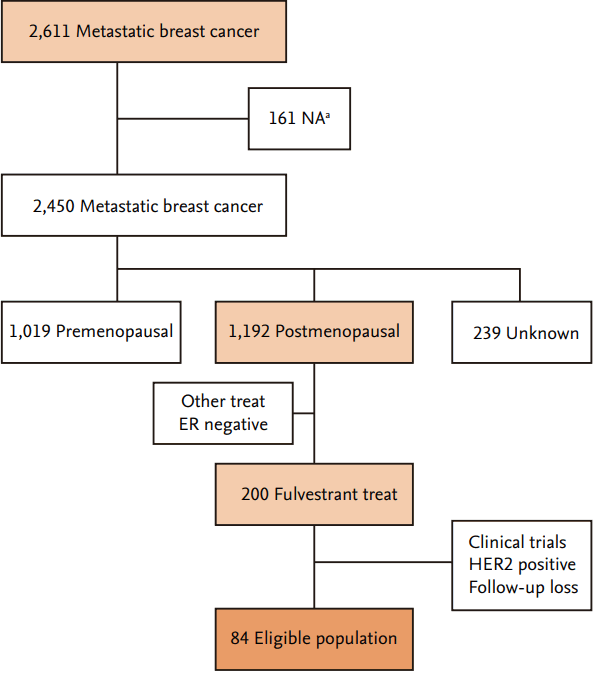
Flow diagram of patient inclusion in this study. NA, not available; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2. a Follow-up loss, double primary cancer, male.
The patient baseline characteristics are summarized in Table 1. The median patient age was 57.7 years (range, 31 to 82). All patients had been treated with at least one endocrine treatment before fulvestrant therapy, including tamoxifen, anastrozole, letrozole, or exemestane. A median of two previous endocrine treatments had been given; 79% of the patients had received two or more endocrine treatments. Forty-five patients (54%) were treated with systemic chemotherapy for MBC, with a median of two chemotherapy regimens. All patients had cancer metastasis and visceral disease was present in 49 patients (58%). Of the patients with visceral disease, 10 had multiple visceral organ involvement. The most common organ of visceral metastasis was the lung, which was present in 34 patients. The other visceral metastases were as follows: liver (n = 16), brain (n = 4), bone marrow (n = 4), adrenal gland (n = 4), and other organs (n = 5) such as pancreas, kidney, and ovary. Regarding non visceral disease (n = 35), 17 patients had bone-only metastasis, 11 had both node and bone metastasis, and seven patients had soft tissue metastasis.
Clinical outcomes and safety
All analyzed patients received fulvestrant for a median treatment time of 3.2 months (range, 1 to 41). The median follow-up duration was 36.4 months (95% CI, 25.2 to 47.6). The estimated median PFS and OS were 4.4 months (95% CI, 3.4 to 5.5) and 32.5 months (95% CI, 17.6 to 47.4), respectively (Fig. 2). The DCR was 40.5% (95% CI, 30.5 to 51.5); PR was observed in 16% and SD in 25% of all patients. Among patients with PR (n = 13, 16%), the response duration was 17.1 months (95% CI, 12.4 to 21.9). The details of these 13 patients are shown in Table 2.

Clinical outcomes of fulvestrant in postmenopausal women previously treated with endocrine therapy (n = 84). (A) Progression-free survival (PFS) of patients treated with fulvestrant (median PFS, 4.4; 95% confidence interval [CI], 3.4 to 5.5 months), (B) overall survival (OS) of patients treated with fulvestrant (median OS, 32.5; 95% CI, 17.6 to 47.4 months).
The therapy-related adverse events are summarized in Table 3. The most frequently reported adverse reactions were mild-to-moderate grade myalgia (10.5% of the patients), injection site pain (7%), and fatigue (7%). Fulvestrant was generally well tolerated. Dose reduction was not required in any of the patients, and no patient discontinued fulvestrant due to adverse events. There were no therapy-related deaths.
DISCUSSION
ER-positive breast cancer represents the most common subtype in both premenopausal and postmenopausal women. AIs and tamoxifen are currently the preferred first-line therapy for ER-positive postmenopausal women with MBC. In the second-line setting, tamoxifen and AIs are used sequentially. Several clinical trials of second-line therapy in metastatic or locally advanced breast cancer for postmenopausal women have shown similar efficacy of these therapies compared to other endocrine therapies, with good tolerability profiles [6,7,13]. The effectiveness of fulvestrant 250 mg was determined by comparing the results for ORR and TTP to those of anastrozole 1 mg. The median TTP was 5.5 months in the fulvestrant arm and 4.1 months in the anastrozole arm (HR, 0.95; 95% CI, 0.82 to 1.10; p = 0.48) and the ORR was 19.2% and 16.5% for fulvestrant and anastrozole, respectively (95% CI, 2.27 to 9.05; p = 0.31). There was no statistically significant difference in OS between the two treatment groups after a follow-up duration of 27 months [6,13]. In the EFECT trial, the effectiveness of fulvestrant 250 mg was compared with that of exemestane. The median TTP was 3.7 months in both groups (HR, 0.96; 95% CI, 0.82 to 1.13; p = 0.653), whereas the ORR was 7.4% and 6.7% in the fulvestrant arm and in the exemestane arm, respectively (HR, 1.12; 95% CI, 0.58 to 2.19; p = 0.736) [7].
The regulatory authorities of many countries have approved fulvestrant for use in MBC after progression on previous endocrine treatment. However, in South Korea, patients are not reimbursed for the use of fulvestrant for ER-positive breast cancer in any situation. We focused on the clinical activity of fulvestrant in postmenopausal women with ER-positive MBC who were treated with endocrine therapy and/or chemotherapy in real clinical practice. In our study the median patient age was 57.7 years (range, 31 to 82); the peak age range of breast cancer incidence in Korea is 45 to 49 years, followed by 50 to 59 years [14]. The DCR was 40.5% (95% CI, 30.5 to 51.5), PR was observed in 16% of the patients, and SD was observed in 25% of the patients, with a mean response duration of 17.1 months (95% CI, 12.4 to 21.9). The therapy-related adverse events were tolerable; however, the toxicity profiles might be underestimated due to the retrospective nature of this analysis. Our results are consistent with existing literature suggesting that fulvestrant is an effective second-line therapy for metastatic or locally advanced breast cancer in postmenopausal women. Moreover, approximately 79% of the patients in our study had undergone two or more endocrine treatments and 54% had been treated with systemic cytotoxic therapy. This finding suggests that fulvestrant has therapeutic efficacy as a salvage treatment in postmenopausal women who have been heavily treated. Due to the high cost of fulvestrant, it is necessary to better define the subgroup of patients who truly benefit from fulvestrant treatment. We found that age, presence of visceral disease, stage at diagnosis, prior adjuvant endocrine therapy, prior cytotoxic therapy, time to surgery to recurrence, and time from first-line endocrine therapy to fulvestrant did not significantly influence clinical outcomes (Supplementary Tables 1 and 2).
Our study has some limitations, including its small sample size, its retrospective nature, and the heterogeneous treatment schedules. These limitations might have affected the findings of this study. In addition, the doses of fulvestrant were not consistent: before November 2010, 24 patients received a fulvestrant dose of 250 mg intramuscularly every 28 days because optimal dose of fulvestrant has not been identified. In our study, the dose of fulvestrant did not influence PFS or OS (PFS: 4.4 months for the 250 mg dose vs. 4.0 months for the 500 mg dose, p = 0.938; OS: 34.7 months vs. 22.7 months, respectively, p = 0.566). However, these findings should be interpreted with caution. In the final report of the CONFIRM (Comparison of Faslodex in Recurrent or Metastatic Breast Cancer) trial, fulvestrant 500 mg was associated with a 19% reduction in risk of death and a 4.1-month difference in median OS compared with fulvestrant 250 mg in patients with locally advanced or metastatic ER-positive breast cancer. Fulvestrant 500 mg was well tolerated, and no new safety concerns were identified [15]. Fulvestrant also showed encouraging clinical activity and favorable feasibility in postmenopausal women with MBC who had been treated with multiple endocrine therapies and/or cytotoxic chemotherapies.
KEY MESSAGE
1. Fulvestrant showed encouraging clinical activity and favorable feasibility in postmenopausal women with metastatic breast cancer who had been treated with multiple endocrine therapies and/or cytotoxic chemotherapies.
2. Fulvestrant was generally well tolerated in patients with heavily treated metastatic breast cancer.
Notes
No potential conflict of interest relevant to this article was reported.