Duodenal Niemann-Pick C1-like 1 expression was negatively correlated with liver X receptor expression in nonalcoholic fatty liver disease
Article information
Abstract
Background/Aims
Intestinal cholesterol absorption includes intestinal Niemann-Pick C1-like 1 (NPC1L1) and is an important target pathway in nonalcoholic fatty liver disease (NAFLD). We investigated the expression of NPC1L1 and its correlation with liver X receptor (LXR) expression in peripheral mononuclear (PMN) cells in patients with NAFLD.
Methods
We evaluated intestinal expression of NPC1L1 in 25 NAFLD patients and 28 healthy controls. We calculated the mRNA expression levels of LXR and farnesoid X receptor (FXR), which are master players of cholesterol metabolism in PMN cells. The protein expression of ABCA1, ABCG5/8, NPC1L1, SREBP, LXR, FXR, and CD36 was measured on tissue samples from the duodenum and ileum.
Results
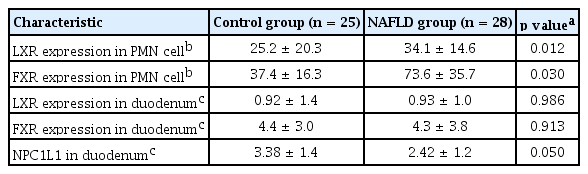
The expression of LXR (p = 0.01) and FXR (p = 0.03) in PMN cells was increased in the NAFLD group compared to the control group. Duodenal NPC1L1 decreased in the NAFLD group compared to the healthy controls (3.38 ± 1.4 vs. 2.42 ± 1.2, p = 0.05). NPC1L1 expression in the duodenum was negatively correlated with LXR expression in PMN cells. Expression of LXR and FXR in the ileum was also negatively correlated with the expression of LXR in PMN cells.
Conclusions
Duodenal NPC1L1 expression was decreased in NAFLD and was negatively correlated with LXR expression in PMN cells.
INTRODUCTION
Hepatic steatosis is mainly due to an imbalance of de novo lipogenesis and fatty acid catabolism. Cholesterol homeostasis is a highly regulated balance of de novo synthesis, dietary cholesterol absorption, fat oxidation, and biliary excretion. Although the roles of cholesterol biosynthetic and clearance pathways are well-known, the uptake and control mechanisms of cholesterol remain largely unknown in nonalcoholic fatty liver disease (NAFLD) [1,2]. The Niemann-Pick C1-like 1 (NPC1L1) protein has been identified as a central player in cholesterol homeostasis. NPC1L1 is expressed in the small intestine, most likely in the brush border membrane of enterocytes, and is required for intestinal cholesterol absorption [3,4]. NPC1L1 was also identified as the molecular target of ezetimibe, the first drug to specifically inhibit intestinal cholesterol absorption [5]. The NPC1L1 knockout mouse improved intrahepatic steatosis and activity of the insulin signaling pathway in the Zucker obese mouse [6]. Although NPC1L1 is a promising target in NAFLD, most clinical trials have not succeeded in reducing the quantity of hepatic fat or intrahepatic inflammation. There are no human studies on expression of NPC1L1 in patients with NAFLD. Moreover, the metabolic signal network is very complex and changes situationally; therefore, NPC1L1 expression should be evaluated in the context of various cholesterol regulators. Liver X receptor (LXR) is another master regulator of hepatic fat homeostasis. In a previous study, we found that hepatic LXR expression correlated with hepatic inflammation and fibrosis in NAFLD patients [7]. At the same time, activation of LXR causes a net loss of cholesterol through the downregulation of NPC1L1 and the induction of ATP-binding cassette transporter (ABCG5/G8 and ABCA1) [8,9]. The correlation between NPC1L1 and LXR, which has a crucial role in hepatic lipogenesis, is very complex. LXR is expressed mainly in the liver, intestine, adipose tissue, and macrophage [10]. Some study proposed that peripheral blood monocytes could be useful to monitor LXR activation [11,12]. Although the roles of LXR and farnesoid X receptor (FXR) in hepatic steatosis are well known, their correlations with LXR/FXR and NPC1L1 have not been thoroughly studied.
We investigated the associations between LXR, FXR, and NPC1L1 as well as their correlation with intestinal cholesterol circulation in patients with NAFLD.
METHODS
Patients and biochemical tests
We evaluated clinical characteristics of 25 NAFLD and 28 control subjects. Inclusion criteria for study subjects include men and women aged between 19 and 75, those who underwent upper endoscopy, colonoscopy, and non-enhanced computed tomography (CT) as part of a routine health screening program and those who voluntarily agreed to participate in this clinical trial, and those who signed a consent form. We excluded all participants who had hepatitis A, B, or C, drug-induced hepatitis, or autoimmune liver disease and those who drink significant quantities of alcohol, which was defined as > 140 g/week in men and > 70 g/week in women. All patients underwent endoscopic biopsy and underwent non-enhanced CT and blood tests on the same day. Fatty liver was diagnosed by decreased liver/spleen (L/S) Hounsfield units. The CT image of NAFLD shows a diffuse decrease in liver density, and the ratio of CT values of the liver and spleen was less than or equal to 1. Patients with a L/S ratio less than 1 were classified as the NAFLD group [13,14]. The study was approved by Eulji Hospital Institutional Review Board (EMCIRB 201505-04) and written informed consent was obtained from each patient. The protocol was registered at the Clinical Research Information Service (http://cris.nih.go.kr/cris/index.jsp) with the registration number KCT0000900. Characteristics (sex; age; body mass index; serum levels of aspartate aminotransferase [AST], alanine aminotransferase [ALT], γ-glutamyl transpeptidase, total cholesterol, triglycerides, low density lipoprotein, high density lipoprotein, albumin, bilirubin, and fasting plasma glucose; platelet count; and prothrombin time) were documented.
Polymerase chain reaction analysis
We calculated the expression levels of LXR and FXR in peripheral mononuclear (PMN) cells by quantitative polymerase chain reaction (qPCR). RNA was isolated from peripheral blood using the Qiamp RNA Blood Mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNA concentration was examined by NanoDrop spectrophotometry (NanoDrop Technologies, Wilmington, DE, USA). Isolated RNA samples were converted to cDNA using reverse transcriptase and oligo (dT) primer (Invitrogen, Carlsbad, CA, USA). Quantitative reverse transcriptase-PCR analysis was performed in the LightCycler 480 system (Roche, Mannheim, Germany) using LightCycler 480 SYBRGreen I Mastermix (Roche). PCR primer sets used were as follows: human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward, 5/-ACCCAGAAGACTGTGGATGG-3/; reverse, 5/-GGATGCAGGGATGATGTTCT-3/; human LXR forward, 5/-GACTGTTCTGTCCCCATATTTTCTG-3/; reverse, 5/-CCCTTCTCAGTCTGTTCCACTTCTA-3/.
Histological assessment of intestine biopsy and tissue microarray construction
Biopsy samples were acquired from the second portion of the duodenum and terminal ileum during gastroduodenoscopy and colonoscopy. Each of the biopsy samples was collected using multiple-sample, single-use biopsy forceps and immediately stored in formalin solution. The biopsy samples were fixed in 10% neutral buffered formalin and embedded in paraffin. The tissue sections were stained with hematoxylin and eosin and reviewed by a pathologist to confirm specimen adequacy. Tissue microarray (TMA) blocks were assembled with the stacking method that we previously described [7]. Briefly, every piece of endoscopic biopsy sample was punched out from each donor block, and excess paraffin was trimmed manually. These tissue cores were stacked on top of one another using a TMA instrument (Quick-Ray, Unitma, Seoul, Korea).
Immunohistochemical staining for various transcriptional factors
Since intestinal cholesterol absorption is tightly regulated by a number of transporter proteins, we evaluated the expression of these transporters in biopsy samples of duodenum and terminal ileum from both NAFLD and control patients by immunohistochemical staining for ABCA1, ABCG5/8, NPC1L1, sterol regulatory element-binding protein (SREBP), LXR, FXR, and CD36. Expression patterns of LXR, FXR, CD36, SREBP-1c, ABCA1, ABCG5, ABCG8, and NPC1L1 were analyzed using anti-human ABCA1 antibody (1:200, NB400-105, Novus, Littleton, CO, USA), anti-ABCG5 antibody (1:200, NBP1-80712, Novus), anti-ABCG8 antibody (1:200, NBP1-83388, Novus), anti-NPC1L1 antibody (1:200, NB400-128, Novus), anti-SREBP-1c antibody (1:200, sc-367, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-LXR-α antibody (1:200, NBP1-77106, Novus), anti-FXR antibody (1:200, sc-13063, Santa Cruz Biotechnology), and anti-CD36 (1:200, Santa Cruz Biotechnology). Then, 4-μmthick tissue sections were cut from the TMA block, deparaffinized, and rehydrated. Heat-induced epitope retrieval was performed with autoclave treatment for 30 minutes in sodium citrate buffer (pH 6.0). Immunohistochemistry staining was carried out using the En-Vision kit (DAKO, Glostrup, Denmark). Endogenous peroxidase activity was blocked with blocking solution (DAKO) at room temperature for 10 minutes. Sections were stained with primary antibodies for 16 hours at 4C°. The tissue sections were then sequentially incubated with ready-to-use horseradish peroxidase immunoglobulin (EnVision/HRP, DAKO) for 30 minutes at room temperature and were developed with 3’-3’ diaminobenzidine as a chromogen substrate. The nuclei were counterstained with Mayer hematoxylin.
Interpretation of immunohistochemical stains
The immunohistochemical expression of intestinal epithelial cells was evaluated by a semi-quantitative scoring method based on staining intensity and the percentage of positive cells. An intensity score was assigned according to the intensity of staining as follows: no staining (0 point), weak staining (1 point), intermediate staining (2 points), and strong staining (3 points). The extent of expression (proportion score) was calculated as the percentage of positively-stained cells in total epithelial cells. The proportion score was assigned according to the staining area as follows: none (0 point), 10% to 24% of the cells (1 point), 25% to 49% of the cells (2 points), 50% to 74% of the cells (3 points), or 75% to 100% of the cells (4 points). A final score was then calculated by multiplying the intensity score by the proportion score.
Statistical analysis
Data are reported as mean ± standard deviation and were analyzed by analysis of variance (ANOVA) and the least significant difference t test using SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA). A value of p < 0.05 was considered significant.
RESULTS
Expression of NPC1L1 and cholesterol transport genes in NAFLD
Body weight, AST, ALT, triglycerides, and γ-glutamylanspeptidase were significantly higher in the NAFLD group than in the healthy controls (Table 1). Expression of LXR and FXR in peripheral blood mononuclear cells was higher in the NAFLD group than in the control group (Table 2). The NAFLD group had higher cholesterol and triglyceride levels than the control group. However, the NAFLD group had lower duodenal NPC1L1 (3.38 ± 1.4 vs. 2.42 ± 1.2, p = 0.05) and FXR expression (6.50 vs. 2.75, p = 0.007) in the terminal ileum than healthy controls (Fig. 1). Duodenal NPC1L1 expression decreased in the presence of high serum triglyceride level (4.96 vs. 3.83, p = 0.03). However, expression of intestinal (duodenum and terminal ileum) LXR and SREBP did not differ between NAFLD patients and controls. Duodenal NPC1L1 was negatively correlated with LXR expression in PMN cells (Fig. 2).

Representative microphotographs of Niemann-PickC1-like 1 (NPC1L1) and farnesoid X receptor (FXR) expressionin the biopsied sample. The duodenal mucosa of a healthycontrol shows an intermediate degree of immunoreactivity forNPC1L1 in the epithelial cytoplasm (A), whereas the mucosaof nonalcoholic fatty liver disease (NAFLD) patents is weaklypositive (B). The ileal mucosa of a healthy control shows diffusestrong nuclear expression of FXR (C), whereas that of the NAFLDpatient shows weak, patch expression (D).
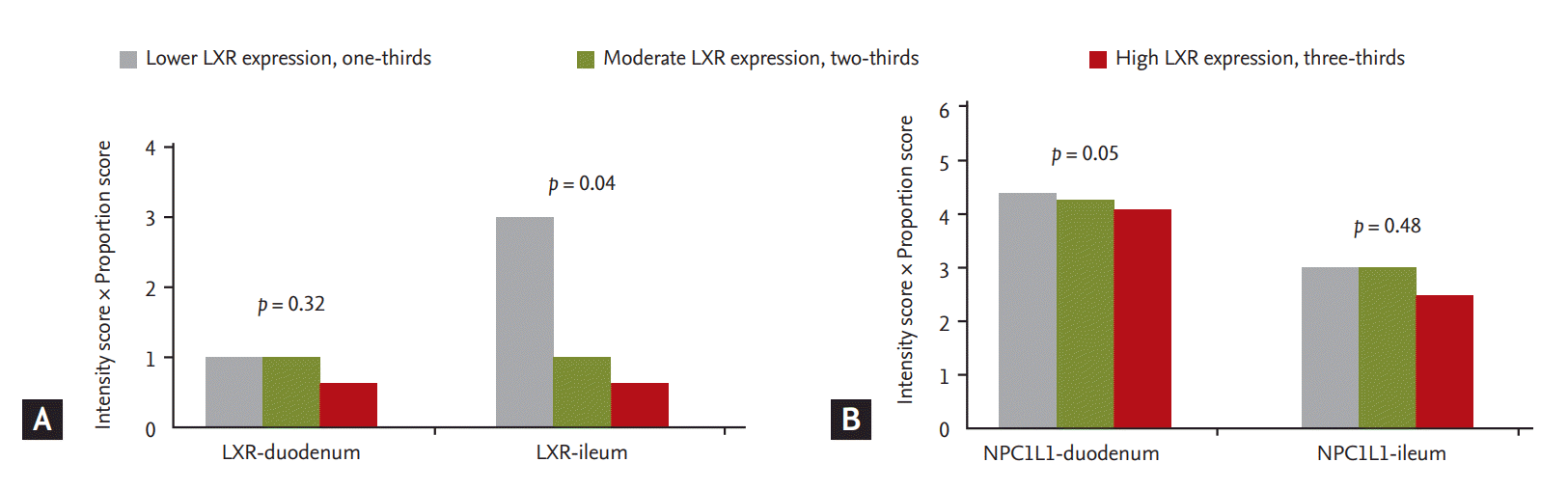
Expression of liver X receptor (LXR) and Niemann-Pick C1-like 1 (NPC1L1) in the duodenum and ileum according toexpression of LXR in mononuclear cells. (A) As expression of LXR in mononuclear cells increased, expression of LXR in theileum decreased. (B) As expression of LXR in mononuclear cells increased, expression of NPC1L1 in the duodenum decreased.
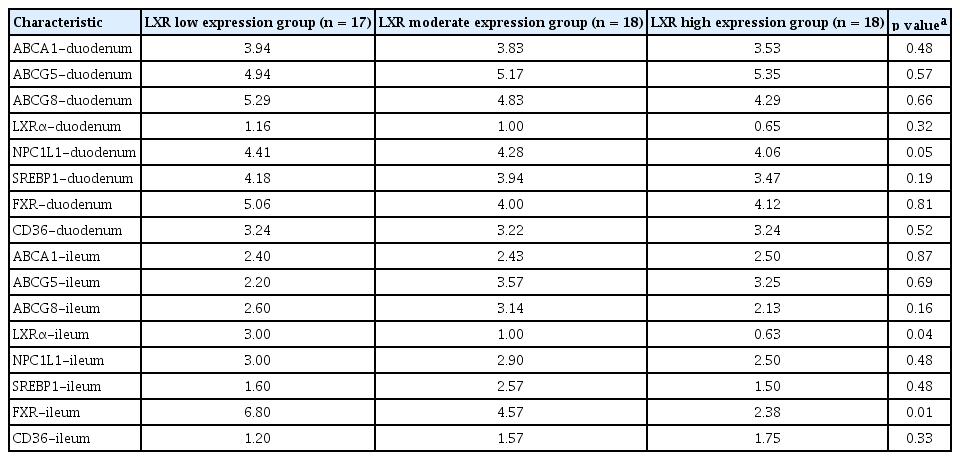
Relationship of LXR expression in PMN cells with gene expression of other cholesterol transport proteins
LXR expression in PMN cells correlated negatively with LXR and FXR in the terminal ileum (Table 3). Increased expression of intestinal LXR was associated with upregulation of ABCA1 (r = 0.40, p = 0.003), ABCG8 (r = 0.25, p = 0.07), FXR (r = 0.60, p = 0.002), and SREBP (r = 0.33, p = 0.016).
DISCUSSION
To our knowledge, there has been no study on the clinical characteristics of NPC1L1 in NAFLD patients. This is the first study to utilize mucosal biopsies from the duodenum and terminal ileum to identify associations between LXR, FXR, and intestinal cholesterol absorption transport proteins in patients with or without NAFLD. We found that LXR and FXR expression was increased in PMN cells in NAFLD patients. Increased LXR, but not FXR, expression was negatively correlated with duodenal NPC1L1 expression. LXR expression in PMN cells might be used as surrogate marker of duodenal NPC1L1 expression.
NPC1L1 has an important role in the absorption and excretion of cholesterol in the apical membrane of the small intestine and the bile canaliculi of the liver. Although more than 50% of NAFLD patients have associated hypercholesterolemia, there is little information on the expression and role of NPC1L1 in NAFLD. Our data showed that cholesterol and triglyceride levels were higher in NAFLD compared to the control group, but the expression of duodenal NPC1L1 decreased paradoxically. This appears to be a homeostatic mechanism of cholesterol absorption and is consistent with a previous study. This study reported that the level of cholesterol absorption from the intestine was inversely related to reverse cholesterol transport from peripheral tissue macrophages into the feces [15]. A Finnish study reported that cholesterol absorption was decreased in NAFLD patients [16]. Ezetimibe is a potent inhibitor of NPC1L1 and is believed to inhibit absorption of cholesterol in the small intestine and excretion of cholesterol in the liver. In a pilot study and an uncontrolled long-term study, ezetimibe improved liver histology and metabolic parameters [17-19]. However, a recent randomized controlled study on NAFLD revealed that ezetimibe did not improve fatty liver [20]. This correlates with the result of our study in that NPC1L1 expression was decreased in the small intestine of patients with fatty liver disease. This suggests that ezetimibe has a very limited effect in patients with decreased NPC1L1 expression, and that specific target populations need to be clarified.
Interestingly, NPC1L1 level in the small intestine and levels of LXR and FXR in peripheral blood were negatively correlated. Animal studies on this relationship have shown that NPC1L1 knockout mice are less sensitive to a synthetic LXR agonist compared to wild-type mice in terms of activation of lipogenic genes; this was supported by hepatic steatosis [21]. NPC1L1 is downregulated via LXR α/β in Caco-2 cells and in the mouse intestine [22]. In another in vivo study, activation of intestinal LXRα reduced cholesterol absorption, increased fecal neutral sterol excretion and m-RCT, and reduced intestinal NPC1L1 mRNA level [23]. LXRα and LXRβ in the enterocyte may compensate for each other in the transcriptional regulation of intestinal NPC1L1. Furthermore, an in vitro study using hepatocytes showed that LXR decreased NPC1L1 in hepatocytes [24]. Our data confirmed that duodenal NPC1L1 expression was negatively correlated with LXR expression in PMN cells in NAFLD. We could estimate duodenal NPC1L1 expression using endoscopic biopsy, but a further large scale study is needed. LXR plays a crucial role in de novo hepatic fat synthesis as well as intrahepatic inflammation [25]. We suggest that the increase in systemic LXR expression in NAFLD patients suppresses intestinal cholesterol absorption. Increased expression of LXR suppresses intestinal expression of NPC1L1 and increases ABC family members ABCA1 and ABCG5/8, which reside on the apical surface of enterocytes and act as efflux pumps moving cholesterol out of absorptive cells into the intestinal lumen. This in turn facilitates cholesterol excretion.
There are some limitations of this study. First, fatty liver was diagnosed by L/S ratio in CT scans and not by biopsy of the liver. Unlike liver biopsy, CT scan cannot detect early cirrhosis or the degree of fibrosis, and it also cannot distinguish NASH from simple steatosis [26]. However, one study suggests that CT scan is superior to ultrasound in detecting hepatic steatosis (82% sensitivity, 100% specificity) [27]. Therefore, it can evaluate the severity of hepatic steatosis, although might not be as accurate as liver biopsy. Second, NPC1L1 expression in the duodenum and terminal ileum might not be generalizable to NPC1L1 expression in the total small intestine. In a study using 11 autopsied human intestinal tissue specimens, NPC1L1 expression showed a bellshaped pattern along the GI tract [28]. However, it might be sufficient to analyze the trend of NPC1L1 expression, as NPC1L1 expression is noted from the duodenum through the colon. Third, other possible factors that might influence cholesterol circulation, such as genetic background, were not investigated in this study. Fourth, we did not show the linear correlation between the expression of NPC1L1 and LXR using Pearson correlation, because of following two reasons. NPC1L1 expression in the duodenum was evaluated by a semi-quantitative scoring method based on staining intensity and the percentage of positive cells. And, LXR expression was evaluated by qPCR. All expressed LXR and FXR values in this manuscript were transformed to log function with LXR and FXR divided into three subgroups and so was the ANOVA.
In conclusion, NPC1L1 expression was decreased in NAFLD patients. Duodenal NPC1L1 expression was lower in patients with hypertriglyceridemia or high LXR expression in PMN cells. Small intestinal NPC1L1 and systemic LXR expression was negatively correlated in patients with NAFLD. Further studies on the use of LXR in peripheral blood as a screening method when using NPC1L1 inhibitors in NAFLD are needed. Research on the relationship between LXR and NPC1L1 might hold promise for the development of novel anti-NAFLD agents.
KEY MESSAGE
1. Niemann-Pick C1-like 1 (NPC1L1) is expressed in the small intestine and is a promising target in nonalcoholic fatty liver disease (NAFLD) treatment. It is known that liver X receptor (LXR) was associated hepatic fat homeostasis.
2. There are no human studies on expression of NPC1L1 in patients with NAFLD.
3. Duodenal NPC1L1 expression was decreased in NAFLD and was negatively correlated with LXR expression in peripheral mononuclear cells.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A121185).