Clinical features and prognoses of acute transverse myelitis in patients with systemic lupus erythematosus
Article information
Abstract
Background/Aims
Acute transverse myelitis (ATM) is a severe complication of systemic lupus erythematosus (SLE). This study evaluated the clinical factors related to outcome in patients with SLE-associated ATM.
Methods
The medical records of patients diagnosed with SLE-associated ATM between January 1995 and January 2015 were reviewed. The patients were divided into two groups based on improvement of neurological deficits after treatment: favorable response group and unfavorable response group. During follow-up, the recurrence of ATM was also analyzed.
Results
ATM was identified in 16 patients with SLE. All of the patients were treated with high doses of methylprednisolone (≥ 1 mg/kg daily). Although 12 patients (75%) recovered (favorable response group), four (25%) had persistent neurologic deficits (unfavorable response group) after the treatment. Compared to the favorable response group, significantly higher Systemic Lupus Erythematosus Disease Activity Index-2000, lower complement levels and initial severe neurologic deficits were found in the unfavorable response group. Among the 12 favorable response patients, five (41.7%) experienced recurrence of ATM during the followup. Patients (n = 5) who experienced relapse had a shorter duration of high-dose corticosteroid treatment (13.2 days vs. 32.9 days, p = 0.01) compared to patients who did not relapse. The mean duration of tapering-off the corticosteroid until 10 mg per day was significantly longer in non-relapse group (151.3 ± 60.8 days) than in relapse group (63.6 ± 39.4 days, p = 0.013).
Conclusions
Higher disease activity in SLE and initial severe neurologic deficits might be associated with the poor outcome of ATM. Corticosteroid slowly tapering-off therapy might be helpful in preventing the recurrence of ATM.
INTRODUCTION
Involvement of the central nervous system is one of the serious complications of systemic lupus erythematosus (SLE), and contributes substantially to the morbidity and mortality [1-4]. Acute transverse myelitis (ATM) is an uncommon spinal cord disorder characterized by the sudden onset and rapid progression of motor, sensory, and autonomic dysfunction [5,6]. This condition is a rare complication of SLE, but is important because it often results in severe irreversible neurological deficits including gait disturbance or voiding difficulty [4].
SLE-associated ATM is usually treated with high doses of corticosteroids alone or in combination with immunosuppressants, such as cyclophosphamide and/or plasmapheresis [7]. However, the optimal treatment of this disease remains largely unknown. Although recurrence of ATM has been reported in approximately 25% of patients with idiopathic transverse myelitis, it is more often found in SLE-associated ATM [4,7]. However, little has been described about the factors associated with the outcomes and recurrence of ATM in patients with SLE. Therefore, in this study, we evaluated the clinical features and treatment outcomes in patients with SLE-associated ATM, and analyzed the factors involved in its prognosis and recurrence.
METHODS
We retrospectively reviewed the medical records of patients diagnosed with SLE-associated ATM at a Asan Medical Center in Korea, from January 1995 to January 2015. All of the patients fulfilled the 1997 revised American College of Rheumatology classification criteria for SLE [8] and the diagnostic criteria for myelopathy in SLE proposed by the American College of Rheumatology in 1999 [3]. The diagnosis of ATM was based on the appropriate clinical features of motor or sensory deficits with or without sphincter dysfunction associated with a spinal cord lesion. Patients with myelopathy related to brain or spinal cord infections, multiple sclerosis, and structural lesions including tumor metastasis, herniated disk, or vertebral fracture were excluded. Demographic characteristics and clinical features such as neurologic symptoms, laboratory, and radiologic findings were collected at the time of diagnosis. Neurological deficits at admission and at the last follow-up were assessed by the American Spinal Injury Association (ASIA) scale, which is stratified from A (no sensory or motor function below the injured spinal level) to E (normal sensory and motor function) [9]. ASIA classification as A, B, or C at presentation was defined as severe myelitis [10]. Treatment modalities and outcomes were evaluated during the follow-up. The Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) was used to evaluate disease activity at ATM diagnosis [11]. The study protocol was reviewed and approved by the Asan Medical Center Institutional Review Board (IRB No. 2015-0349) and performed in accordance with the principles of the Declaration of Helsinki, and informed consents were waived due to this retrospective nature.
Laboratory assessments
Laboratory data including complement (C3, C4, and CH50), anti-double-stranded DNA (anti-dsDNA) antibody titer, serum erythrocyte sedimentation rate, C-reactive protein, and autoantibody profiles were collected. Examination of cerebrospinal fluid (CSF) included analysis of cellularity, protein content, and glucose.
Radiological assessment
We only included patients who had undergone spinal cord magnetic resonance imaging (MRI) on a 1.5-T MR scanner for diagnostic workup. The sequences included sagittal T1- and T2-weighted and axial T2-weighted images. The number, localization, and extent of spinal lesions were documented. Longitudinally extensive lesions were defined as more than three vertebral segment spinal cord lesions on MRI [7].
Treatment outcomes
Treatment regimens, recurrence rates, and death rates were evaluated. Patients were categorized into two groups according to improvement of neurologic deficits after treatment. Patients whose ASIA scale improved after treatment were regarded as the favorable response group (including both partial and complete improvement). The unfavorable response group comprised patients with permanent initial neurologic impairments despite treatment. Among the favorable response group, relapse was defined as recurrence of neurologic symptoms with spinal lesion at MRI.
Statistical analysis
Data are expressed as the mean ± standard deviation or median (interquartile range [IQR]). Categorical data are expressed as absolute number and percentage. Comparison of the frequencies of various findings among the patient groups was performed using the Mann-Whitney U test or the chi-square test. Odds ratios (ORs) were reported with 95% confidence intervals (CIs). The cumulative probability of disease relapse during follow-up was calculated using the Kaplan-Meier method and data were compared using the log-rank test. A p < 0.05 was considered statistically significant. Statistical analyses were performed using the SPSS software version 21.0 (IBM Corp., Armonk, NY, USA).
RESULTS
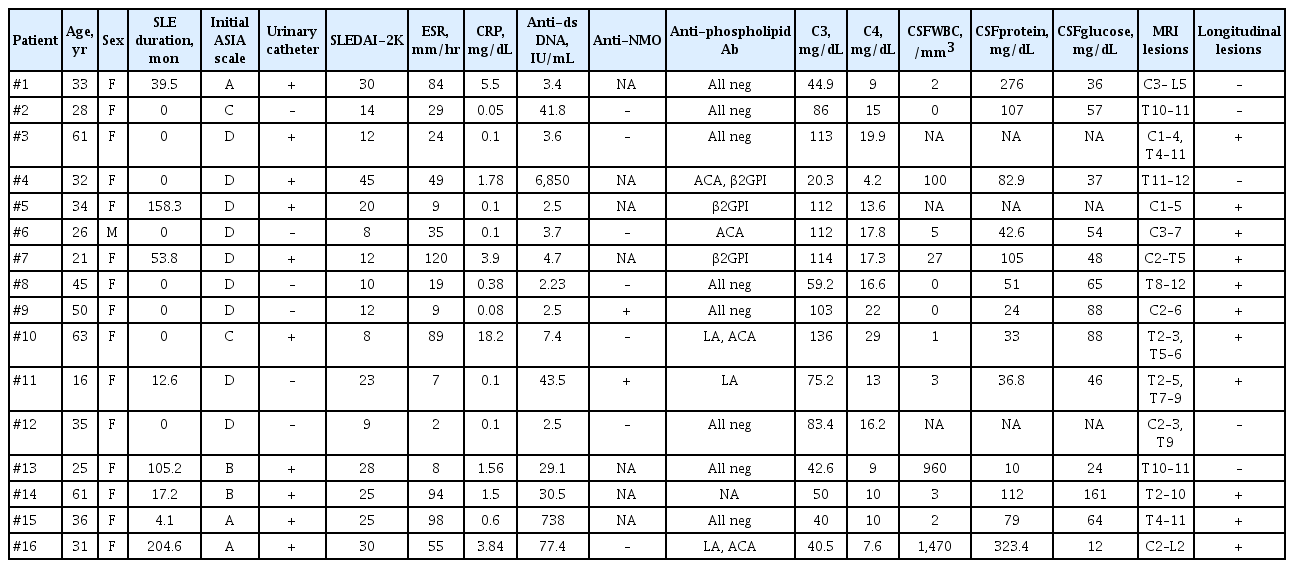
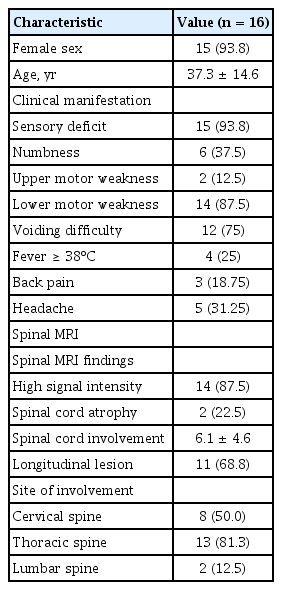
Of the 2,473 patients diagnosed with SLE between 1995 and 2015, 16 (0.64%) were identified as ATM. The clinical characteristics, treatment regimens and outcomes for these patients are described in Tables 1-3. Fifteen of the 16 patients were female, and their mean age at diagnosis of ATM was 37.3 ± 14.6 years. In eight (50%), ATM was identified as an initial manifestation of SLE, and in the remaining eight patients, SLE disease duration prior to the development of ATM was a median of 74.4 months (IQR, 13.7 to 145). Observed neurological manifestations of ATM were sensory deficits (15/16, 93.8%), motor impairment of the lower extremities (14/16, 87.5%), and urinary sphincter dysfunction (12/16, 75%). In total, 10 of the 12 patients with urinary sphincter dysfunction required urinary catheterization. Patients were classified based on their neurological deficits according to the ASIA scale, and three of 16 patients (18.8%) had ASIA scale A; two (12.5%) had ASIA scale B; another two (12.5%) had ASIA scale C; and nine (56.3%) had ASIA scale D. The antiphospholipid antibodies (including Lupus anticoagulant [LA], anti–β2-glycoprotein I [β2GPI], anticardiolipin antibody [ACA]) test was performed in 15 patients. Of the 15 patients, seven (46.7%) were positive for antiphospholipid antibodies. ACA immunoglobulin M (IgM) and/or IgG were found to be positive in four patients (26.7%). LA and anti-β2GPI antibodies were detected in 20.0% (3/15) and 20.0% of patients (3/15) in the tested population, respectively. Three patients were positive for more than two antiphospholipid antibodies. The CSF examinations of the patients are listed in Table 1. Three of total 16 patients didn’t undergo CSF examination. Pleocytosis, defined as white blood cell count above 10/mm3 in CSF analysis, was found in four of 13 patients. Eight had the levels of protein greater than 50 mg/dL in CSF examination.
A total of 87.5% patients (14/16) showed a high signal intensity of the spinal cords on T2-weighted images, and 22.5% patients (2/16) showed spinal cord atrophy. Eleven had longitudinal transverse myelitis. The most common site of involvement was the thoracic spine (n = 13), followed by the cervical spine (n = 8) and the lumbar spine (n = 2).
All of the patients were initially treated with high doses of methylprednisolone (≥ 1 mg/kg/day). The median time from onset of initial neurological symptoms to high-dose corticosteroid treatment was 15.5 days (IQR, 5.5 to 52.5). Fifteen patients (93.8%), eight patients (50.0%), two patients (12.5%), and one patient (6.25%) were treated with methylprednisolone pulse therapy, cyclophosphamide pulse therapy, plasmapheresis and rituximab therapy, respectively.
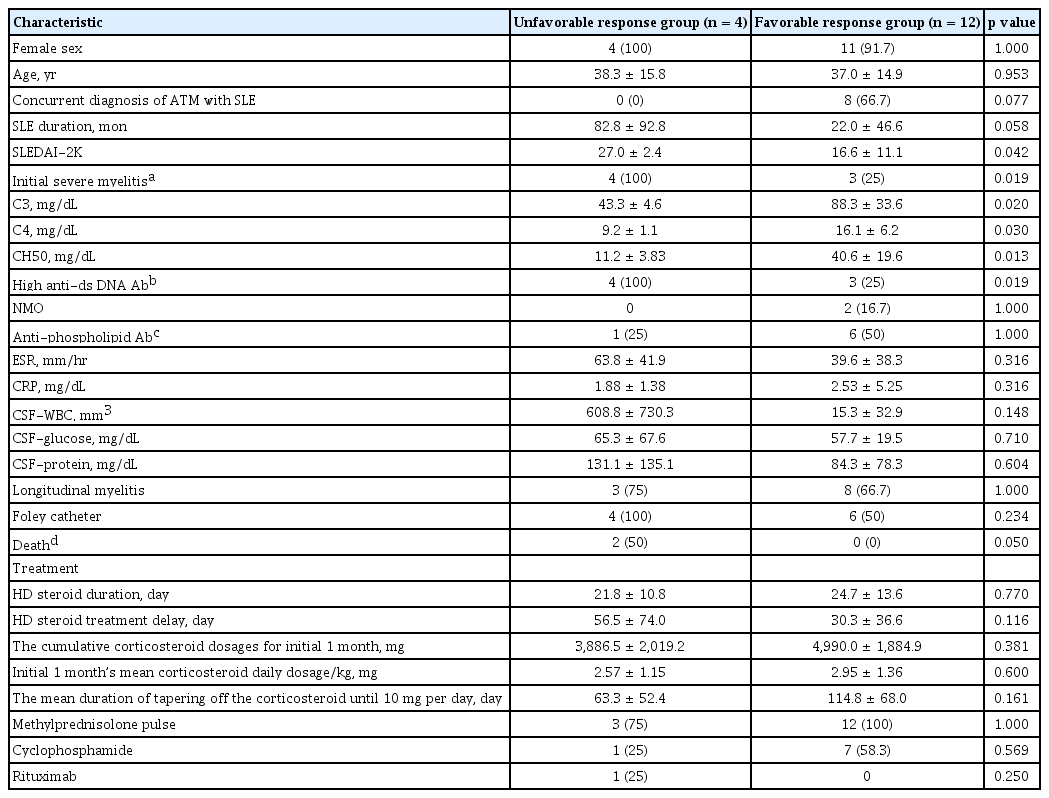
The median follow-up period was 6.3 years (IQR, 2.0 to 7.6). Analyses of 16 patients were performed, of which 12 (75%) belonged to the favorable response group, including 10 patients with complete recovery. Four patients (25%) belonged to the unfavorable response group, and included those who had no improvement in their neurological symptoms at the last follow-up. The mean time until recovery of motor function was 53 days (range, 4 to 180). Baseline demographic data and clinical characteristics between the two groups of patients at the time of diagnosis are shown in Table 4. Gender and age did not differ between groups, and there were no significant differences in high-dose corticosteroid treatment delay. The cumulative corticosteroid dosages for initial 1 month and initial 1 month’s mean corticosteroid daily dosage per kg (body weight) were 3,886.5 ± 2,019.2 mg and 2.57 ± 1.15 mg/kg in unfavorable response group, 4,990.0 ± 1,884.9 mg and 2.95 ± 1.36 mg/kg in favorable response group. Eight out of sixteen patients were treated with cyclophosphamide pulse therapy. Cyclophosphamide pulse therapy had no significant influence on neurologic outcome.
Patients diagnosed with SLE before ATM onset showed a three-fold higher risk (OR, 3.0; 95% CI, 1.34 to 6.67) of developing unfavorable neurologic response compared to those who presented with ATM as the first manifestation of SLE.
Higher SLEDAI-2K scores were found in the unfavorable response group compared to the favorable response group (27.0 ± 2.4 vs. 16.6 ± 11.1, p = 0.042). Severe myelitis (identified as ASIA A–C) at baseline was significantly more frequently observed in the unfavorable response group (p = 0.019). Furthermore, serum complement levels were significantly lower in the unfavorable response group than in the favorable response group (C3, p = 0.020; C4, p = 0.030; CH50, p = 0.013, respectively). Also, high titer (> 20 IU/mL) of anti-dsDNA antibody was shown in more patients in unfavorable response group than in favorable response group. Neuromyelitis optica (NMO)-IgG antibodies were tested in the serum of nine patients, and were positive in two patients. Three patients, including two with NMO-IgG, had optic neuritis during the course of ATM. During the follow-up, two patients (12.5%) died, both of whom were in the unfavorable response group (p = 0.05).
Of the 12 favorable response patients, five (41.7%) experienced at least one recurrence of ATM during the follow-up (median follow-up duration until relapse, 10.4 months; IQR, 4.4 to 48.4; range, 2 to 68) (relapse group). The remaining seven patients (58.3%) did not experience relapse (non-relapse group). The patients (n = 5) who experienced relapse had a shorter duration of high-dose corticosteroid treatment (13.2 days vs. 32.9 days, p = 0.01) than the patients who did not relapse (Table 5). Relapse was more frequent in the shorter duration (< 2 weeks) of high-dose corticosteroid treatment group than in the longer duration (≥ 2 weeks) of high-dose corticosteroid treatment group (Fig. 1). The mean duration of tapering-off the corticosteroid until 10 mg per day was significantly longer in non-relapse group (151.3 ± 60.8 days) than in relapse group (63.6 ± 39.4 days, p = 0.013).
DISCUSSION
There have been few studies about the clinical characteristics and prognostic factors of SLE patients with ATM. Previous studies reported an incidence of ATM of 1.34 to 4.6 per million per year [12,13]. Prevalence of ATM in SLE patients is higher than general population [4,6]. Also, the incidence of ATM in our study was 0.64% (16 of 2,473 patients).
In our present study, patients presenting with severe neurologic deficits at ATM onset demonstrated a higher frequency of poor neurologic outcomes than those with mild neurologic deficits at baseline. Significantly lower levels of complements (C3, C4, and CH50) were detected in ATM patients with an unfavorable response. Furthermore, a high SLEDAI-2K scores were associated with poor neurologic outcome. These findings suggest that severe disease involvement including SLE activity is associated with unfavorable neurologic outcomes in ATM. Previous researches have shown that the factors associated with poor neurologic outcomes include urinary sphincter dysfunction [14], initial severe neurological impairment [10,15], and extensive spinal cord lesions [16]. Thus, it appears that disease severity at initial presentation can influence the outcome of SLE-associated ATM [4,15].
Results of the CSF examination in our patients were quite diverse. Usually, SLE-associated ATM has an inflammatory CSF finding which shows pleocytosis and elevated protein levels [15,17]. According to some investigators, however, analysis of the cerebrospinal fluid finding does not manifest any abnormalities [18,19], others show diversity like our study [20].
Patients in our current series who had a longer duration of SLE before ATM onset demonstrated a higher frequency of unfavorable neurologic responses with an OR that was approximately 3-fold higher than that of patients who presented with ATM as the first manifestation of SLE. There have been no previous studies on neurologic outcome according to duration of SLE, although a Swedish study in patients with neuropsychiatric SLE reported that those presenting with severe neurologic manifestations had a longer SLE disease duration [21].
It is reported that the presence of antiphospholipid antibodies is highly associated with SLE-associated ATM [22]. It is known that the prevalence of having antiphospholipid antibodies is 18% to 60% of patients with SLE-associated ATM [4,14,17]. There is still debate about the relevance of status of antiphospholipid antibodies and prognosis of SLE-associated ATM [14,15,17]. In the current study, seven of 15 patients (46.7%) had antiphospholipid antibodies and there was no significant association between positivity of antiphospholipid antibodies and prognosis of ATM.
High-dose corticosteroid therapy is the main treatment option for myelopathy in SLE. Although there is no standard duration of treatment, high doses of corticosteroids followed by intravenous cyclophosphamide are recommended for myelopathy in SLE [23]. Several studies have reported that treatment delay (> 2 weeks) is a predictor of poor outcome in SLE-associated ATM [4,24]. In our present study, the time lag between neurologic symptom onset and administration of high-dose corticosteroid in the favorable group did not significantly differ from that in the unfavorable response group, possibly because the patients who had severe neurologic symptoms (identified as ASIA A–C) received high-dose corticosteroid therapy earlier than those who had mild neurologic symptoms (median lag time, 9.3 days vs. 19.6 days).
A recurrence of ATM was observed in five patients. In patients with relapse, the interval between initial ATM onset and relapse ranged from 2 to 68 months. It is more likely for ATM to recur if patients are treated for less than 2 weeks with high dose corticosteroid. Treatment of high dose corticosteroid for over 2 weeks might be helpful in preventing the relapse. In addition, it took significantly longer to taper off the corticosteroid until 10 mg per day in non-relapse patients than in relapse patients. Therefore, the present findings suggest that corticosteroid slowly tapering-off might is helpful in preventing ATM relapse.
NMO, also termed Devic syndrome, is a clinicopathological entity characterized by optic neuritis, TM, and positive NMO-IgG autoantibody [23]. In some patients, it may be associated with SLE-associated ATM (~20%) [10]. Of the 16 patients assessed in our present study, two were thought to have concurrent NMO according to the revised 2006 NMO classification with clinical findings predictive of ATM relapse [25]. Given that relapses are more frequent in patients with NMO [4,26], it is interesting that in our current analysis, two patients with NMO relapsed twice during the follow-up.
The main limitations of our present study include its small sample size and retrospective design. Another limitation is that our study population comprised Asian patients only. Despite these shortcomings, our results may help clinicians make treatment decisions about SLE patients with ATM.
In conclusion, the majority of patients with SLE-associated ATM experience favorable neurologic outcomes after high-dose corticosteroid treatment, but some have permanent neurologic deficits and recurrence. Our data suggest that high SLE disease activity and initial severe neurologic deficits might be associated with the poor neurologic outcome of ATM. Corticosteroid slowly tapering-off therapy and long-term treatment with high doses of corticosteroids (> 2 weeks) might be helpful in preventing the recurrence of ATM.
KEY MESSAGE
1. Higher disease activity in systemic lupus erythematosus and initial severe neurologic deficits might be associated with the poor outcome of acute transverse myelitis.
2. Corticosteroid slowly tapering-off therapy might be helpful in preventing the recurrence of acute transverse myelitis.
Notes
No potential conflict of interest relevant to this article was reported.