New insights into the genetics of fibromyalgia
Article information
Abstract
Although debate on the concept of fibromyalgia (FM) has been vigorous ever since the classification criteria were first published, FM is now better understood and has become recognized as a disorder. Recently, FM has come to be considered a major health problem, affecting 1% to 5% of the general population. As familial aggregations have been observed among some FM patients, genetic research on FM is logical. In fact, genome-wide association studies and linkage analysis, and studies on candidate genes, have uncovered associations between certain genetic factors and FM. Genetic susceptibility is now considered to influence the etiology of FM. At the same time, novel genetic techniques, such as microRNA analysis, have been used in attempts to improve our understanding of the genetic predisposition to FM. In this article, we review recent advances in, and continuing challenges to, the identification of genes contributing to the development of, and symptom severity in, FM.
INTRODUCTION
A considerable proportion of the general population suffers from pain symptoms; population-based studies have shown that 10% to 55% of the population experience diverse chronic pain symptoms at any given time [1]. Fibromyalgia (FM) is a common and complex chronic pain syndrome that affects 1% to 5% of the population [2]. FM is now defined as chronic widespread pain (CWP), persisting for more than 3 months, without any obvious organic lesion. FM is commonly accompanied by additional symptoms, such as joint stiffness, fatigue, sleep disturbance, cognitive dysfunction, and depression [3,4]. Although debate on the concept of FM has continued ever since the time of first publication of the classification criteria, FM is now better understood and is generally recognized as a disorder [5].
Patients with FM exhibit mental and physical disabilities and a significantly impaired quality-of-life [6]. FM imposes a major strain on healthcare resources, accompanied by high disease-related medical costs [7,8]. As FM is now recognized as a major health problem, many investigators have sought to explore the pathophysiology of FM. Although the etiology of FM remains unclear, significant advances have been made in our understanding of the pathophysiology of FM. The recognition of central sensitization is representative of these advances, providing an evidence-based explanation for chronic pain, including that of FM [9]. Central sensitization refers to blunting of inhibitory pain pathways and alterations in neurotransmitter levels, leading to abnormal processing of sensory signals within the central nervous systems (CNS), eventually lowering the threshold of pain and amplifying the sensations from normal signals, causing chronic pain [10].
Currently, the pathophysiology of FM is considered to involve the interaction of several factors, including abnormalities in the neurobiological and autonomic nervous systems, genetic factors, psychological variables, and environmental factors [11]. In this article, we review the current explanations for the pathophysiology of FM, focusing on genetic factors involved in the development of, and symptom severity in, FM.
EVIDENCE FOR A GENETIC PREDISPOSITION TO FIBROMYALGIA
Genetic factors contributing to chronic pain
Pain commonly aggregates in families, and previous studies have shown that heritability explains up to 50% of the development of chronic pain [12]. Given such work [13], a revolution in molecular genetics followed, suggesting that some forms of chronic pain have genetic explanations.
The search for pain-related genes has primarily featured large linkage or association analyses; these showed that pain-related genes affected the expression or function of proteins in a manner influencing the pain response [12]. Currently, hundreds of pain-regulated genes that are thought to be relevant to pain perception or analgesia have been identified. These include the genes encoding voltage-gated sodium-channels (Nav), GTP cyclohydrolase 1 (GCH1), mu-opioid receptors, and catechol-O-methyl transferase (COMT); and various genes of the dopaminergic, glutamatergic, and GABAergic pathways [14]. Furthermore, numerous candidate genes have been identified from gene expression profiling (microarray) studies [15].
Familial aggregation of fibromyalgia
As the role played by genetic factors in pain mechanisms became clearer, much research effort focused on a possible genetic predisposition to FM. Familial aggregation among FM patients provided reasonable support for an association between genetic factors and the development of FM [16]. Pellegrino et al. [17] evaluated 17 families of FM patients to assess the evidence that FM might be an inherited condition. Although the study had a relatively small sample size, it was suggested that the mode of FM inheritance was autosomal-dominant. It was found that 26 (52%) of the enrolled parents and siblings exhibited clinical evidence of FM, and an additional 11 (22%), without apparent symptoms of FM, exhibited abnormal muscle consistency on palpation. Similar observations in terms of familial aggregation among FM patients were also reported in two studies by Buskila et al. [18] and Buskila and Neumann [19]. In one study, among 58 offspring of 20 complete nuclear families of FM-diagnosed mothers, 16 (28%) were found to have FM. As the exposure of offspring to environmental factors, and psychological and familial factors, did not differ between those with and without FM, it was concluded that the high-level familial occurrence of the condition might be attributable to genetic factors [18]. Buskila and Neumann [19] further studied female FM patients and their close family members, such as blood relatives (parents, siblings, and children), and non-related members (husbands). The prevalence of FM among the blood relatives of FM patients was 26%, whereas that among non-related members was only 19%. Furthermore, pain sensitivity was measured using mean tender point counts. The pain sensitivities of young male and female relatives were significantly higher than those of controls. These two studies thus suggested that certain genetic factors were associated with pain sensitivity and the high-level familial occurrence of FM. Arnold et al. [20] also evaluated the familial aggregation of FM and pain sensitivity among 533 relatives of 78 probands with FM. The presence of FM was associated both with significant aggregation and higher tender point counts among the relatives of FM probands compared with those of rheumatoid arthritis probands. Another study by Arnold et al. [21] showed that the sibling recurrence risk for FM was 27.2% (95% confidence interval [CI], 22.5 to 31.9), yielding a sibling recurrence risk ratio of 13.6 (95% CI, 10.0 to 18.5).
Moreover, FM patients exhibit familial aggregations of psychological features, such as depression and personality traits; such findings also support the idea that genetic factors contribute to the development of FM. Glazer et al. [22] assessed shared personality traits in FM patients and their first-degree relatives. Personality traits were evaluated (using the Tridimensional Personality Questionnaire [TPQ]) in 129 female FM patients, 27 female relatives with undiagnosed FM, and 30 female relatives without FM. FM patients and relatives with FM exhibited higher scores on the ‘harm avoidance’ subscale than did relatives without FM. Although it was not possible to fully differentiate between genetic and nongenetic factors, it was concluded that hereditary factors for personality traits contributed to the development of FM.
The fact that familial aggregation and shared psychological traits are evident among patients with FM does not exclude roles for other non-genetic factors in the pathogenesis of FM. However, the findings described above do suggest that genetic factors make important contributions to the etiology of FM.
GENOME-WIDE ASSOCIATION STUDIES IN FIBROMYALGIA
Based on the observational findings of familial studies, subsequent research was devoted to identification of relevant genetic mutations using various research tools. The identification of genetic risk factors can now be achieved using many different technologies, study designs, and analytical tools. Among these techniques, genome-wide association studies (GWAS) are a relatively new way to identify susceptibility genes involved in human disease, and the technique is attractive when it is sought to explore the genetic architecture or heritability of certain human conditions [23]. Feng et al. [24] performed whole-exome sequencing and subsequent directed mutation analysis to discover possible candidate genes for FM. The cited authors identified two nonsense mutations associated with high levels of specific cytokines. The W32X mutation in C11orf40 and the Q100X mutation in in ZNF77 (zinc finger protein 77) correlated with high plasma MCP-1 (monocyte chemoattractant protein 1) and IP-10 (interferon γ-induced protein 10) levels, and with high plasma IL-12 (interleukin 12) levels, respectively. These findings suggested that inflammation, including deregulation of the expression of specific cytokines, might be associated with the development of FM. Docampo et al. [25] also explored the genetic susceptibility to FM via GWAS and copy number variant (CNV) analyses. Although no single-nucleotide polymorphism (SNP) (of more than 5 × 105 SNPs assessed) attained the genome-wide significance threshold, 21 of the most highly associated SNPs were further replicated in 952 FM cases and 644 controls. Replication analysis identified two associated variants, an SNP in the MYT1L (myelin transcription factor 1 like) gene and an intronic CNV in the NRXN3 (neurexin 3) gene, suggesting a potential role for CNS dysfunction in FM.
Recently, Peters et al. [26] performed the first GWAS meta-analysis on 1,308 females with CWP, and 5,791 controls of European descent, and replicated the effects of the genetic variations, deriving suggestive evidence for associations in 1,480 CWP cases and 7,989 controls. The work identified a common genetic variant rs13361160 on chromosome 5p15.2, associated with CWP, located upstream of the chaperonin-containing-TCP1-complex-5 gene (CCT5) and downstream of FAM173B. Although this large genetic study targeted CWP, thus not specifically FM, the work identified promising genetic targets for study of the pathogenesis of FM.
LINKAGE STUDY IN FIBROMYALGIA
A genetic linkage study uses recombinant genetic technology to map genetic loci, based on the observation that genes that are physically close on a given chromosome typically remain linked during meiosis [27]. Similar to GWAS, linkage analysis can be used to identify regions of the genome that contain genes predisposing to certain diseases. Arnold et al. [21] performed the first genome-wide linkage scan for FM in a cohort of multicase families. The cited authors genotyped members of 116 families, evaluating 341 markers, and detected a signal in the chromosome 17p11.2‑q11.2 region, which coincided with the map coordinates of the serotonin transporter gene (SLC6A4) and the transient receptor potential (TRP) vanilloid 2 (TRPV2) genes. Although subsequent sequencing analyses of these chromosome regions in FM cases were not performed, the work does suggest that the two genes are candidate FM genes.
CANDIDATE GENE STUDIES IN FIBROMYALGIA
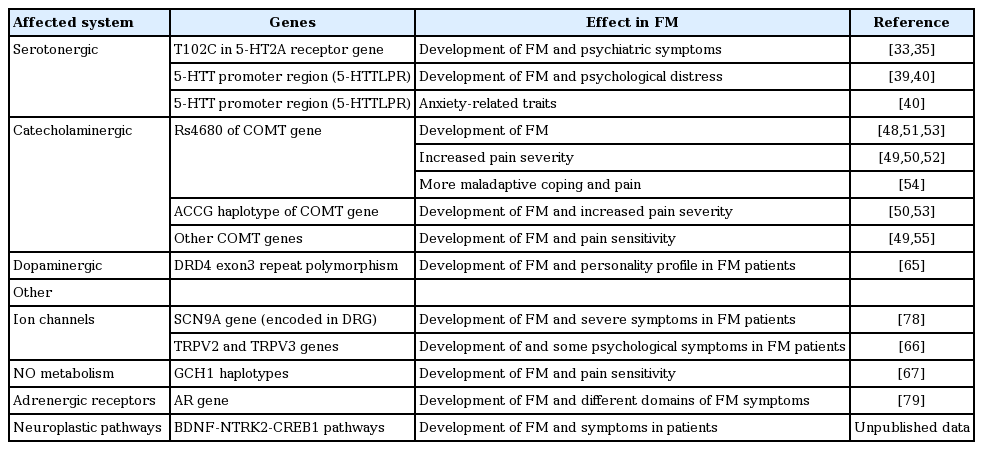
The results of GWAS and linkage studies have expanded our understanding of the genetic basis of FM, and have suggested that several genetic markers may be FM susceptibility genes. At the same time, studies targeting potential candidate genes considered likely to be associated with the pathogenesis of FM have been performed by several investigators (Table 1). Although the data are not yet sufficient, these studies have advanced our knowledge of genetic susceptibility to FM. Associations between FM and certain genetic polymorphisms affecting the serotonergic, dopaminergic, and catecholaminergic pathways have been found via candidate gene analyses.
Genes affecting serotonergic metabolism
Recent studies found that FM was associated with disturbances in serum and cerebrospinal fluid (CSF) serotonin metabolism and neurotransmission; the levels of 5-HT and metabolites thereof were significantly lower in the serum and CSF of FM patients [28-30]. The serotonin system is regulated primarily by the 5-hydroxytryptamine receptor 2A (5-HT2A) and the serotonin transporter (5-HTT) [31]. 5-HT2A is found widely throughout the CNS and is encoded by the HTR2A (5-hydroxytryptamine receptor 2A) gene. 5-HTT is also known as the sodium-dependent serotonin transporter and solute carrier family 6 member 4, and is encoded by the SLC6A4 gene, suggested to be an FM candidate gene in one linkage study [21]. The 5-HTT regulates the transport of serotonin from synaptic spaces into presynaptic neurons, playing an important role in serotonergic neurotransmission [32]. Thus, genetic mutations affecting both serotonin receptors and 5-HTT have received attention as possible susceptibility genes in terms of the pathophysiology of FM.
In this context, Bondy et al. [33] evaluated the association between a silent polymorphism (T102C) in the 5-HT2A receptor gene and FM. The cited authors found a significant difference in the genotype distribution in FM patients; the T/T genotype was decreased and both the T/C and C/C genotypes were increased, compared with controls. Moreover, pain symptoms were significantly higher among patients with the T/T genotype. Mergener et al. [34] also suggested that the T102C polymorphism was a genetic susceptibility factor for FM. However, Gursoy et al. [35] found no difference in the level of the T102C polymorphism between FM patients and controls. However, the genotype was associated with symptom severity. The T/T genotype was associated with psychiatric symptoms and lower pain thresholds in FM patients. Tander et al. [36] reported that polymorphisms in 5-HT2A seemed not to contribute to susceptibility to FM. Nevertheless, a recent meta-analysis concluded that the 5-HT2A receptor T102C polymorphism did confer susceptibility to FM [37]. However, other serotonin receptor subunit genes, such as HTR3A and HTR3B, were not associated with the development of FM [38].
Some investigators have also evaluated the association between the promoter region of the 5-HTT gene and the development of FM. Offenbaecher et al. [39] showed that the frequency of the S/S 5-HTT genotype was higher among FM patients than controls. Furthermore, the S/S genotype was associated with severe symptoms of depression and other forms of psychological distress. Similarly, Cohen et al. [40] found an association between FM and a 5-HTT promoter region polymorphism. Also, the short allele of the polymorphism was associated with anxiety-related traits. However, Gursoy [41] reported that neither the polymorphism in the 5-HTT promoter region nor the variable number of tandem repeats (VNTR) variant was associated with susceptibility to FM in patients of normal psychiatric status. Moreover, no association between the 5-HTT promoter region polymorphism and FM was observed in a meta-analysis [37].
Thus, to date, any role of serotonergic gene polymorphisms in the pathophysiology of FM remains inconclusive. Nevertheless, clinical experience with 5-HT3 receptor antagonists and re-uptake inhibitors used to treat FM has provided supporting evidence indicating that genes affecting the serotonergic pathway can serve as genetic markers of FM [42,43]. The serotonergic pathway influences diverse psychiatric symptoms, such as depression, anxiety, and fatigue, which are common in patients with FM [44]. Thus, we cannot exclude an indirect effect of such gene polymorphisms on FM. Further studies are needed to understand their significance more fully.
The COMT gene
Catechol-O-methyl transferase is a major enzyme inactivating catechol-containing drugs and catecholamine neurotransmitters such as dopamine, epinephrine, and norepinephrine. The COMT enzyme is expressed in the CNS, where it affects catecholamine neurotransmission in the prefrontal cortex [45]. Various mutations in the COMT gene induce functional enzyme impairments [46]. Thus, SNPs of the COMT gene have been suggested to contribute to FM susceptibility and symptom severity.
One mutation commonly studied in the context of FM pathophysiology is the Val158Met polymorphism (SNP rs4680). This SNP is associated with a valine (Val GTG) to methionine (Met ATG) transition at codon 158 [47]. This Met substitution reduces the activity of the COMT enzyme. In fact, the H/H (GG; Val-158-Val) genotype generates a fully effective enzyme, but the H/L (AG; Met-158-Val) genotype an intermediate-activity enzyme. The L/L (AA; Met-158-Met) genotype produces a defective enzyme, which impairs the clearance of catecholamines from the CNS. A significant association between this SNP and FM development has been observed in subjects of diverse ethnic backgrounds. Matsuda et al. [48] and Barbosa et al. [49] found associations between rs4680 and FM susceptibility in a Brazilian population. Similar associations were found in a Spanish population by Vargas-Alarcon et al. [50]; in a Turkish population by Gursoy et al. [51]; and in an Israeli population by Cohen et al. [52]. Furthermore, the role of rs4680 was not limited to FM susceptibility; the SNP also affected symptom severity. Barbosa et al. [49] showed that rs4680 was associated with increased pain severity as measured by the Fibromyalgia Impact Questionnaire (FIQ) score. In another study, Martinez-Jauand et al. [53] found that FM patients with the Met158Met genotype exhibited higher sensitivity to painful thermal and pressure stimuli than did patients with the Val158Met genotype. Finan et al. [54] showed that COMT rs4680 played a role in daily maladaptive coping and pain in FM patients. In this study, FM patients with the Met158Met genotype reported more maladaptive coping and higher-level pain than did those with the Val158Val genotype. However, others failed to find any association between this SNP and FM development. Studies in various ethnic populations revealed no association between rs4680 and FM development [36,55]. We evaluated the association between COMT SNPs and FM susceptibility in a Korean population, using a dataset of the Korean Nationwide FM Survey to evaluate genetic factors in, and clinical manifestations and outcomes of, FM [56]. In that largescale study, we found that rs4860 was not associated with FM susceptibility. Additionally, an earlier meta-analysis revealed no association between the rs4680 SNP and FM risk [57,58]. Furthermore, in a recent meta-analysis, stratification by ethnicity failed to show any association between rs4680 and FM in European or Turkish populations [57]. Thus, evidence for an association between rs4680 and FM susceptibility remains inconclusive; further large, well-designed studies are needed.
Other COMT gene polymorphisms, including the SNPs rs6269, rs4633, and rs4818, have also been evaluated in FM patients [49,50,53]. In a Korean population, we found that rs4818 and rs4633 may be associated with FM risk and pain sensitivity [56]. However, the roles of these SNPs in FM remain poorly understood. In addition, certain haplotypes have attracted the attention of investigators. Apart from individual SNPs, specific haplotypes of the COMT gene have been associated with pain sensitivity (individual responses to various stimuli) [59]. One particular COMT gene haplotype (ACCG), producing a defective enzyme, was associated with more sensitive perception of painful stimuli. Thus, the ACCG haplotype has been defined as a high-pain-sensitivity haplotype. Indeed, Vargas-Alarcon et al. [50] revealed associations among the ACCG haplotype, the presence of FM, and higher FIQ scores in FM patients.
Collectively, the COMT gene is the most widely investigated candidate gene in terms of FM susceptibility. Despite the variations observed among different ethnic populations, the COMT gene remains an attractive genetic target in studies of FM. To confirm the role played by the COMT gene in FM, further prospective studies are needed. The effects of various polymorphisms on the disease course and outcomes require evaluation.
Genes affecting the dopaminergic pathway
Although dopamine is well-known to play roles in the sensation of pleasure, reward-motivation behavior, and motor control, recent studies suggest that dopamine is also involved in modulating pain perception and natural analgesia [60]. Recent work has shown that dopamine plays an important role in the descending inhibition of pain [61]. Based on this finding, researchers have shown that FM patients exhibit disrupted dopaminergic reactivity to painful stimuli [62,63]. Moreover, indirect evidence from a previous clinical trial also suggested a role for dopamine in the pathophysiology of FM. Pramipexole, a dopamine 3 receptor agonist, was analgesic in FM patients compared with placebo [64]. For these reasons, dopaminergic genes have been suggested as potential contributors to FM genetic susceptibility. In fact, a D4 dopamine receptor exon III repeat polymorphism has been associated with the personality profile of FM patients [65]. FM patients with this polymorphism scored poorly on the TPQ novelty-seeking personality subtest. However, to date, any role for dopaminergic genes in FM remains inconclusive. Further large-scale studies are needed.
Candidate genes from the Korean Nationwide FM Survey
As part of the Korean Nationwide FM Survey, we evaluated genetic factors that might be associated with FM susceptibility and symptom severity (Table 2). We performed relatively large-scale candidate gene studies, involving more than 400 FM cases and 400 controls, to overcome the problem with the low sample sizes of previous studies. As mentioned above, we found that COMT gene polymorphisms were associated with FM risk in a Korean population, and suggested that ethnic variation in COMT gene polymorphisms might play a role in FM [56]. Moreover, based on the findings of a recent linkage study suggesting that the TRPV2 gene was a candidate FM gene [21], we investigated the association between polymorphisms in the TRPV2 and TRPV3 genes, FM susceptibility, and symptom severity [66]. The frequencies of the alleles and genotypes of individual TRPV2 and TRPV3 genes did not differ significantly between FM cases and controls. However, we found that certain TRPV2 haplotypes may play a protective role in FM and that some genotypes and haplotypes of TRPV3 may contribute to the symptoms of FM. Given the evidence that TRPV channels contribute to pain signaling pathways, such as peripheral and central sensitization, the TRPV gene may be a valuable target for future genetic research on FM.
Apart from the TRPV gene, Kim et al. [67] evaluated associations of GCH1 gene polymorphisms with FM. The GCH1 gene encodes an enzyme termed GTP cyclohydrolase 1, which is the rate-limiting enzyme in the synthesis of tetrahydrobiopterin (BH4) [68]. BH4 is an essential cofactor for nitric oxide (NO) production, and increased NO levels enhance pain sensitivity. Furthermore, earlier research showed that a polymorphism in the GCH1 gene was associated with reduced pain sensitivity [69]. Kim et al. [67] showed that the CCTA haplotype of the GCH1 gene was associated with protection against FM susceptibility, and significantly lowered the pain sensitivity of FM patients. Collectively, as NO metabolism is involved in various processes, including neurotransmission and immune regulation [70], these findings suggest that NO may also be involved in the altered pain perception of FM.
Using data from unpublished works, we found that polymorphisms in the brain-derived neurotrophic factor (BDNF) and the adenosine monophosphate response element-binding protein (CREB1) genes were associated with susceptibility to FM. The BDNF and CREB1 genes may be candidate FM genes. BDNF is responsible for modulation of nociceptive inputs and enhances hyperalgesia via the N-methyl-D-aspartate (NMDA) receptor [71,72]. Binding of BDNF to its receptor (neurotrophic tyrosine kinase receptor, type 2 [NTRK2]) triggers phosphorylation of the nuclear transcription factor CREB1 [73]. The BDNF-NTRK2-CREB1 pathways have been evaluated in terms of responses to antidepressants and the pathophysiology of depression [74], which is commonly associated with FM. Thus, our results suggest that these neuroplastic pathways may afford hidden links to the development of FM.
Other candidate genes
Smith et al. [75] performed a large-scale candidate gene analysis using a dedicated gene-array chip (the Pain Research Panel), which assays variants of over 350 genes known to be involved in biological pathways relevant to nociception, inflammation, and affect. After replication analysis using an independent cohort, the authors suggested that the trace amine-associated receptor 1 (TAAR1), regulator of G protein signaling 4 (RGS4), cannabinoid receptor 1 (CNR1), and glutamate ionotropic receptor AMPA type subunit 4 provided (GRIA4) genes were potentially associated with the development of FM. Interestingly, previous candidate genes, including COMT SNP rs4680 and the dopamine transporter gene, were not identified in this study. Nevertheless, this research suggested potential therapeutic targets for FM.
Several ion channels have attracted attention as gates for the detection and processing of thermal, mechanical, and chemical pain stimuli [76]. For example, various sodium channelopathies have been proposed as risk factors for pathological pain conditions [77]. In FM, Vargas-Alarcon et al. [78] found that patients with polymorphisms in the sodium channel SCN9A gene, expressed in the dorsal root ganglia, had higher FIQ scores. Furthermore, Vargas-Alarcon et al. [79] found an association between adrenergic receptor (AR) gene polymorphisms and FM. The AR gene polymorphisms were associated with a risk of developing FM and with the scores on different domains of the FIQ. Based on the roles played by AR polymorphisms in symptoms related to relentless sympathetic hyperactivity, which is frequently observed in FM patients [80], the AR gene is also a reasonable candidate gene for FM.
MICRORNA ANALYSES
The pathophysiology and symptoms of FM are not fully explained by the genetic factors described in this review. Thus, there is still a need to evaluate other possible pain-related genes. In concert with genetic studies on FM, novel genetic techniques including microRNA analyses have been used to assess the regulation of gene expression in FM. Bjersing et al. [81] conducted the first microRNA study, identifying nine microRNAs that were significantly lower in the CSF of FM patients than controls. In another study, Bjersing et al. [82] identified eight serum-circulating microRNAs that were differentially expressed between FM patients and healthy female controls. In both microRNA studies, it was first found that certain microRNAs were associated with symptom severity in FM patients [81,82]. These results suggest that new genetic research tools will advance our understanding of the genetic susceptibility to FM.
CONCLUSIONS
Currently, FM is considered to result from an interaction between genetic factors and environmental factors. Earlier researchers discovered evidence for familial aggregation of FM, supporting the likelihood of a genetic background to the disease. Based on these findings, more recent research has focused on genetic features triggering alterations in gene expression in those with FM. This work has revealed that certain polymorphisms in genes involved in the serotonergic, dopaminergic, and catecholaminergic pathways may be involved in FM development. Furthermore, these polymorphisms influence not only susceptibility to FM but also symptom severity.
However, the genetic factors identified to date do not fully explain the etiology of FM. The genetic studies conducted over the past two decades have not completely explained the molecular mechanisms of FM. Moreover, the effects of genetic factors on FM disease progression, therapeutic response, and outcomes have not yet been defined. Further studies are required to evaluate the relationships between genetic polymorphisms and these clinical aspects of FM. Indeed, more prospective studies, using various genetic techniques such as GWAS, linkage analysis, candidate gene analysis, and microRNA studies, are needed to deepen our understanding of the genetic basis of FM.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
We thank the patients and their families for their participation. We also thank the doctors and nurses who joined the Korean Nationwide FM Survey. This study was supported by a grant (CRI16015-1) Chonnam National University Hospital Biomedical Research Institute and by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (2017M3A9E8023014).