Notch signaling in the collecting duct regulates renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction in mice
Article information
Abstract
Background/Aims
Mind bomb-1 (Mib1) encodes an E3 ubiquitin ligase, which is required for the initiation of Notch signaling. Recently, it was demonstrated that the renal collecting duct plays an important role in renal fibrosis. Here, we investigated the role of Notch signaling in renal fibrosis using conditional knockout mice with the specific ablation of Mib1 in renal collecting duct principal cells.
Methods
Mib1-floxed mice (Mib1f/f) were crossed with aquaporin 2 (AQP2)-Cre mice in order to generate principal cell-specific Mib1 knockout mice (Mib1f/f :AQP2-Cre+). Unilateral ureteral obstruction (UUO) was performed, and mice were sacrificed 7 days after UUO.
Results
After performing the UUO, renal tubulointerstitial fibrosis and the expression of transforming growth factor β were markedly enhanced in the obstructed kidneys of Mib1f/f mice compared with the sham-operated kidney of Mib1f/f mice. These changes were shown to be even more pronounced in the obstructed kidneys of Mib1f/f :AQP2-Cre+ mice than in those of the Mib1f/f mice . Furthermore, the number of TUNNEL-positive cells in renal collecting duct was higher in the obstructed kidneys of Mib1f/f :AQP2-Cre+ mice than in the kidneys of Mib1f/f mice.
Conclusions
Notch signaling in the renal collecting duct plays an important role in the regulation of renal tubulointerstitial fibrosis and apoptosis after UUO.
INTRODUCTION
Progressive renal tubulointerstitial fibrosis occurs in virtually every type of chronic kidney disease, and it is characterized by excessive accumulation of fibroblasts/myofibroblasts with the increased production and deposition of extracellular matrix (ECM), including collagen, fibronectin, and other related fibrogenic molecules [1-3]. Although the activation of fibroblasts/myofibroblasts is generally considered the main cause of the excessive production and deposition of ECM in the pathogenesis of renal tubulointerstitial fibrosis, the underlying mechanisms remain unclear [4,5].
The Notch signaling pathway is an evolutionary conserved cell-cell communication mechanism. Four receptors, Notch1–4, and five ligands, delta-like1, 3, 4 (Dll1, 3, 4), and Jagged1 and 2 (Jag1, 2), involved in this signaling pathway can be found in mammals [6]. The endocytosis of Notch ligands in the signal sending cells is necessary for the initiation of notch signaling. In mammals, four E3 ubiquitin ligases, neuralized-1 (Neur1) and Neur2, and mind bomb-1 (Mib1) and Mib2, are known to regulate the endocytosis of Notch ligands [7]. Among these E3 ubiquitin ligases, only Mib1 has an obligatory role in the activation of Jag- as well as DII-mediated Notch signaling in mammalian development, while Neur1, Neur2, and Mib2 activity is dispensable [8,9]. Thus, genetic mutation of Mib1 represents an excellent model for the elucidation of the role of Notch signaling [10,11]. In kidney diseases models, Notch signaling pathway is reported to be involved in renal fibrosis-related diseases, such as diabetic nephropathy and focal segmental glomerulosclerosis [12]. Notch signaling pathway plays an important role in the development of tubulointerstitial fibrosis as well [13]. Several studies showed that the principal cells of renal collecting duct may play a role in the development of tubulointerstitial fibrosis [14-17]. However, their role remains unclear [17]. Unilateral ureteral obstruction (UUO) is the most widely used animal model of chronic kidney disease, with renal tubulointerstitial fibrosis [18,19].
In this study, we determined the role of Notch signaling in principal cells of renal collecting duct in renal tubulointerstitial fibrosis induced by UUO. Principal cell-specific Mib1 knockout mice that underwent UUO surgery were used in our experiments.
METHODS
Animals
To generate mice with a Mib1 deletion specifically in principal cell of collecting duct (Mib1-floxed mice [Mib1f/f]:aquaporin 2 [AQP2]-Cre+), we crossed Mib1f/f mice (kindly provided by Prof. Kong, Seoul National University, Seoul, Republic of Korea) with AQP2-cre mice (Stock No. 006881, purchased from The Jackson Laboratory, West Grove, PA, USA). All mice were crossed on a C57BL6 background and only male mice were used in the study. UUO was performed as described previously [18]. Briefly, mice were anesthetized with zoletil and the left ureter was exposed via a left dorsal incision. The mid-ureter was then obstructed using a two-point ligation with silk sutures. The sham-operated mice underwent the same procedure with the exception of obstruction of the left ureter and used as controls. Mice were sacrificed at 7 days after UUO. After anesthetized, the animals were perfused with phosphate buffered saline (PBS; pH 7.4), and then fixed with 2% paraformaldehyde-lysine-periodate solution, which was administered through the heart for 10 minutes. After perfusion, the kidneys were removed and cut into 1 to 2 mm thick slices, which were further fixed by immersion in the same fixative overnight at 4˚C. All the experimental procedures were performed according to the Animal Care and Ethics Legislation and the study was approved by the Animal Care Committee of Bucheon St. Mary’s Hospital.
Antibodies
The antibodies used in this study were as follows: AQP2 (Millipore, Billerica, MA, USA), Notch1 (Abcam, Cambridge, UK), fibronectin (DAKO, Glostrupp, Denmark), collagen IV (SouthernBiotech, Birmingham, AL, USA), fibroblast-specific protein 1 (FSP1, Thermo Scientific, Cheshire, UK), transforming growth factor β (TGF-β, R&D systems, Minneapolis, MN, USA), Smad4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), c-Myc (Santa Cruz Biotechnology), and glyceraldehyde 3-phosphate dehydrogenase (Santa Cruz Biotechnology) were used. Apoptosis was detected using an ApopTag Peroxidase in situ Apoptosis Detection Kit (Millipore).
Immunohistochemical analysis
For single post-embedding immunohistochemical staining (IHC), after fixation, kidney was embedded in wax and cut transversely at a thickness of 4 μm using a microtome. Some kidney sections were processed and stained with periodic acid-Schiff (PAS) stain or Masson’s trichrome stain. Other sections were processed for post-embedding immunohistochemistry analysis. These tissue sections were hydrated with graded ethanol and rinsed in tap water. After dewaxing, the sections were incubated with retrieval solution for 10 minutes by microwave and then washed in tap water. They were incubated with methanolic H2O2 for 30 minutes for endogenous peroxidase blocking. After the process, they were incubated with 0.5% Triton X-100/PBS solution for 15 minutes and they were rinsed with PBS. The nonspecific binding sites were blocked with normal donkey serum diluted 1:10 in PBS for 1 hour and subsequently incubated overnight with a primary antibody at 4˚C. Next day, after rinsing in PBS, the sections were incubated for 2 hours in peroxidase-conjugated donkey anti-mouse or anti-rabbit immunoglobulin G (IgG; Jackson Immuno Research Lab., West Grove, PA, USA) and washed again with 0.05 M Tris buffer (pH 7.6). For detection, the sections were treated with 0.05% 3,3’-diaminobenzidine (DAB) and 0.01% H2O2 mixture. The sections were washed with distilled water, dehydrated with graded ethanol and xylene, mounted in Canada balsam, and examined by light microscopy.
For multiple post-embedding IHC, after DAB colorizing, tissue sections were treated with methanolic H2O2 for 30 minutes to remove any peroxidase remaining from the first staining. The sections were incubated with the other primary antibody. After a wash in PBS, the sections were incubated for 2 hours with peroxidase-conjugated donkey anti-rabbit IgG (Jackson Immuno Research Lab.). For detection of peroxidase, Vector SG (Vector Laboratories, Burlingame, CA, USA) was used as a chromogen to produce a grayish blue color, which is easily distinguished from the brown staining produced by DAB. The sections were washed with distilled water, dehydrated with graded ethanol and xylene, mounted in Canada balsam, and examined by light microscopy.
Western blot analysis
The kidney was homogenized in boiling lysis buffer (1% sodium dodecyl sulfate [SDS], 1 mM sodium orthovanadate, and 10 mM Tris, pH 7.4) and the protein concentration was determined with the BCA Protein assay kit (Pierce Biotechnology Inc., Rockford, IL, USA). Equal amounts of the protein were separated on SDS-polyacrylamide gel. The gel was transferred onto a nitrocellulose membrane. For immunodetection, the nonspecific binding sites were blocked with PBS that containing 0.1% Tween-20 and 5% skim milk and then the blots were incubated overnight in the same solution with the primary antibody. The blots were washed and then incubated with a secondary antibody conjugated to horseradish peroxidase (Jackson Immuno Research Lab.) and the blots were visualized using a Western blotting luminol reagent kit (Santa Cruz Biotechnology).
Cell counting and statistics
The deposition of immunohistochemistry results and cell counting were measured by JNOPTIC Image Analysis Software OpTIC Eye (Seoul, Korea). The values are expressed as percentage of the total number of cells in the respective segments. Values are presented as the mean ± SE. Data were compared between groups using an unpaired t test and Microsoft Excel 2007 (Microsoft, Redmond, WA, USA). p values less than 0.05 were considered significant.
RESULTS
The expression of Notch1
The functional role of Notch signaling in the principal cells of renal collecting duct in renal tubulointerstitial fibrosis, was investigated by generating principal cell-specific conditional Mib1 knockout mice (Mib1f/f:AQP2-Cre+).
First, we investigated Notch 1 expression in sham-operated and UUO groups. The number of Notch 1-positive cells in Mib1f/f:AQP2-Cre+ mice sham-operated mice was lower than that in Mib1f/f mice. Its expression was higher in tubular epithelial cells after UUO. The number of Notch 1-positive cells in Mib1f/f:AQP2-Cre+ mice was lower than in Mib1f/f mice after UUO (Fig. 1A). Following this, we performed double IHC staining using antibodies for AQP2 (marker of the principal cells of collecting duct) and Notch 1. In both sham-operated control mouse kidneys and the obstructed kidneys, Notch 1 expression in AQP2-positive principal cells was considerably lower in Mib1f/f:AQP2-Cre+ mice than in Mib1f/f mice (Fig. 1B), suggesting that the Notch signaling pathway is selectively inhibited in the principal cells of Mib1f/f:AQP2-Cre+ mouse collecting ducts.

(A) Notch 1 expression and the quantification of Notch 1-positive cells. Scale bars: 100 µm. The results are presented as mean ± standard error. (B) Aquaporin 2 (AQP2, blue) and Notch 1 (brown) sample staining. Scale bars: 50 µm. Mib1f/f, mind bomb-1 (Mib1)-floxed mice; UUO, unilateral ureteral obstruction. a p < 0.05 vs. Mib1f/f Sham, b p < 0.05 vs. Mib1f/f UUO.
Principal cell-specific Mib1 deletion enhances tubulointerstitial fibrosis after UUO
The effects of principal cells-specific deletion of Mib1 in renal tubulointerstitial fibrosis induced by UUO were investigated by PAS and Masson’s trichrome staining. ECM deposition within the tubulointerstitium at day 7 after UUO was higher in Mib1f/f mice than in sham-operated Mib1f/f mice, and the deposition was even greater in the obstructed kidneys of Mib1f/f:AQP2-Cre+mice (Fig. 2). Similar data were obtained by analyzing fibronectin expression using Western blot (Fig. 3A). The expression of type IV collagen was higher in the obstructed kidneys of Mib1f/f:AQP2-Cre+ mice than in those of Mib1f/f mice (Fig. 3B). Furthermore, we performed the IHC staining for FSP1, as a marker of fibroblast. FSP1 expression was significantly increased in the obstructed kidneys of Mib1f/f:AQP2-Cre+ mice compared with that in the Mib1f/f mice (Fig. 3C).

(A) Periodic acid-Schiff and (B) Masson’s trichrome staining of the investigated samples. Scale bars: 50 µm. Mib1f/f, mind bomb-1 (Mib1)-floxed mice; AQP2, aquaporin 2; UUO, unilateral ureteral obstruction.

(A) Representative fibronectin (FBN) immunoblot results. (B) Collagen IV immunostaining and the quantification of collagen IV deposition (mask area/field area %). Scale bars: 50 µm. (C) Fibroblast-specific protein 1 (FSP1) immunostaining and the quantification of FSP1 deposition (mask area/field area %). Scale bars: 100 µm. The results are represented as mean ± standard error. Mib1f/f, mind bomb- 1 (Mib1)-f loxed mice; AQP2, aquaporin 2; UUO, unilateral ureteral obstruction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. a p < 0.05 vs. Mib1f/f Sham, b p < 0.05 vs. Mib1f/f UUO.
Principal cell-specific Mib1 deletion induces the expression of TGF-β1 and Smad4
TGF-β and Smad signaling pathways are important mechanisms in renal fibrosis. IHC analyses showed that TGF-β1 expression was upregulated in the obstructed kidneys of Mib1f/f:AQP2-Cre+ mice after UUO (Fig. 4A). Western blot analyses revealed that TGF-β1 and Smad4 protein expression levels were considerably higher in the obstructed kidneys of Mib1f/f:AQP2-Cre+ mice than in the kidneys of Mib1f/f mice (Fig. 4B and 4C).
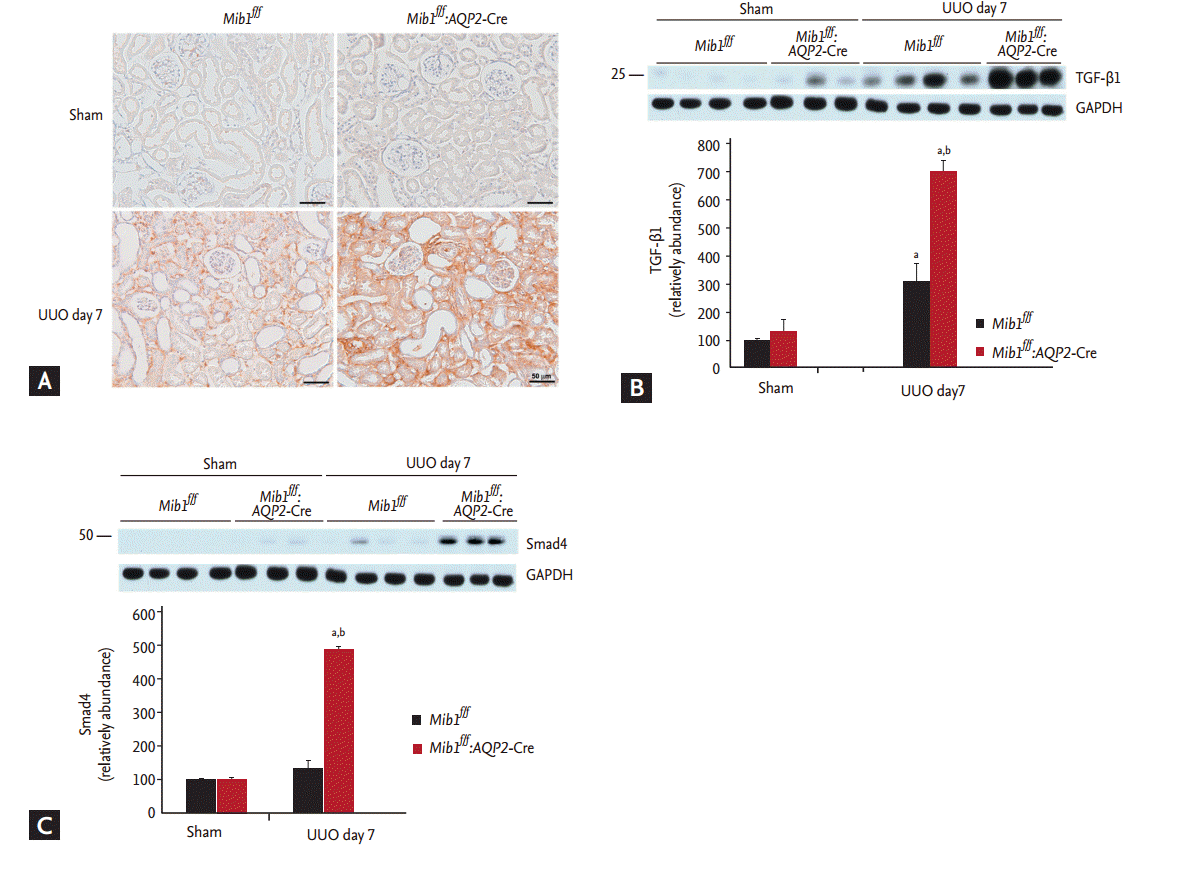
(A) Immunohistochemical staining and representative result of immunoblot analysis showing (B) transforming growth factor β1 (TGF-β1) and (C) Smad4 expression. Scale bars: 50 µm. The results are represented as mean ± standard error. Mib1f/f, mind bomb-1 (Mib1)-f loxed mice; AQP2, aquaporin 2; UUO, unilateral ureteral obstruction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. a p < 0.05 vs. Mib1f/f Sham, b p < 0.05 vs. Mib1f/f UUO.
Principal cell-specific Mib1 deletion enhances apoptosis after UUO
The role of Mib1 in cellular apoptosis after UUO was investigated using the TUNNEL (Terminal deoxynucleotide transferase dUTP Nick End Labeling) assay. While a very low number of TUNNEL-positive cells was detected in the sham-operated control mice, this number increased 7 days after UUO and was observed to be even higher in the obstructed kidneys of Mib1f/f:AQP2-Cre+ mice than in the obstructed kidneys of Mib1f/f mice (Fig. 5A).

(A) TUNNEL (Terminal deoxynucleotide transferase dUTP Nick End Labeling) assay results and the quantification of TUNNEL-positive cells, (B) aquaporin 2 (AQP2) (blue) and TUNNEL (brown) double-staining is presented, with TUNNEL positive cell percentage in the collecting duct (%) and TUNNEL-positive cell number in non-collecting duct (/mm2). (C) Analysis of c-Myc expression. Scale bars: 50 µm. The results are presented as mean ± standard error. Mib1f/f, mind bomb-1 (Mib1)-floxed mice; UUO, unilateral ureteral obstruction; CD, collecting duct; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. a p < 0.05 vs. Mib1f/f Sham, b p < 0.05 vs. Mib1f/f UUO.
Additionally, we showed that the number of TUNNELpositive cells in AQP2-positive collecting ducts increased more in Mib1f/f:AQP2-Cre+ mice than in Mib1f/f mice, and it was even higher in Mib1f/f:AQP2-Cre+ mice after UUO. However, the number of TUNNEL-positive cells in the non-collecting duct decreased after UUO in Mib1f/f:AQP2-Cre+ mice (Fig. 5B). The expression of c-Myc was analyzed, and it was a shown to be significantly higher in the obstructed kidneys of Mib1f/f:AQP2-Cre+ mice than in those of Mib1f/f mice (Fig. 5C).
DISCUSSION
Here, we investigated the role of Notch signaling in the collecting duct in renal tubulointerstitial fibrosis. The obtained results demonstrate that Notch signaling in principal cells of the collecting duct plays a role in the development of renal tubulointerstitial fibrosis. The genetic deficiency of Mib1 in principal cells led to the upregulation of TGF-β1 and promoted renal tubulointerstitial fibrosis after UUO. Additionally, the obtained results indicate that the principal cell-specific Mib1 deletion increases ECM deposition and enhances tubulointerstitial fibrosis after UUO. The results obtained by investigating the expression of Notch 1 in Mib1 deficient sham-operated or UUO mice showed that Mib1 is a potential regulator of Notch signaling pathway, and that the induction of Notch 1 is inhibited in mice with principal cell-specific Mib1 deletion.
The precise mechanism of Mib1-mediated regulation of renal tubulointerstitial fibrosis is unclear [19]. Several possible mechanisms have been suggested. UUO may result in a distal nephron injury. Furthermore, UUO may alter the structure and cellular composition of the collecting duct cells, which may result in reduction of AQP2-positive principal cell numbers [19]. Jeong et al. [11] reported that Mib1 deficiency in ureteric bud (Mib1f/f:Hoxb7-Cre+ mice) leads to the inactivation of Notch signaling during the development of renal collecting duct, which inhibits principal cell differentiation from precursor cells and diminishes the number of principal cells. Additionally, Notch signaling pathway was reported to be activated after acute ischemic injury, which requires the regeneration of tubular cells in order to restore the integrity of the tubular epithelium [20]. Therefore, the inactivation of Notch signaling in principal cells of Mib1-deficient mice may lead to an insufficient repair of principal cells after UUO. Furthermore, as recent studies show, using in vitro models [15,16] and fetal UUO model [14], it was shown that collecting duct cells may be involved in the development of renal tubulointerstitial fibrosis.
Here, we showed that the rate and the number of TUNNEL-positive cells after UUO in the collecting duct were significantly higher, whereas the death rate of noncollecting duct cells was lower in the obstructed kidneys of Mib1f/f:AQP2-Cre+ mice than in those of Mib1f/f mice (Fig. 5A and 5B). The c-Myc overexpression may lead to an increase in apoptosis [21-23]. Our finding that c-Myc expression is significantly higher in the obstructed kidneys of Mib1f/f:AQP2-Cre+ mice than in those of Mib1f/f mice may indicate that the apoptotic effect of Mib1 in renal principal cell after UUO may be regulated by c-Myc. The obtained data show that the increase in cell death rate in collecting ducts may be one of the underlying mechanisms of renal tubulointerstitial fibrosis development. Further studies to elucidate the precise mechanism are needed.
The results of this study are inconsistent with a previous study, which reported that genetic deletion and pharmacologic inhibition of the members of the Notch signaling pathway in proximal tubules reduce renal tubulointerstitial fibrosis [13]. Although the reason for this disagreement between the results of this study and the results we obtained is unclear, we suggest that it may be due to the differences in the experimental design. Cellular response after UUO may differ between proximal and distal nephrons [24]. In response to UUO, superoxide molecules accumulate in proximal tubules, causing apoptosis and necrosis, which is followed by progressive atrophy and collapse of proximal tubules. The rapid proximal tubular damage in response to UUO injury may be a primary determinant of renal parenchymal loss, which may result in progressive increase in ECM deposition. In contrast to the proximal tubules, the distal nephrons may have adaptive responses to the UUO injury. In distal nephrons, collecting ducts dilate and undergo cellular remodeling, in order to preserve distal tubular integrity and maintain their patency [24]. In this study, we used the principal cells-specific Mib1 deficient mice, while Bielesz et al. [13] used the proximal tubules-specific Notch 1 deficient mice (Rbpjf/f:PEPCK-Cre+ mice). Notch signaling pathway may play different roles in the segment-specific nephron responses to UUO injury, which should be investigated in further studies.
In conclusion, these results suggest that the inactivation of Mib1, an E3 ligase expressed by the ligand-expressing cells, is required for efficient Notch activation, which leads to increased renal tubulointerstitial fibrosis and apoptosis of principal cells after UUO.
KEY MESSAGE
1. Mind bomb-1 (Mib1) is a potential regulator of Notch signaling pathway.
2. Notch signaling in the collecting duct plays an important role in the regulation of renal tubulointerstitial fibrosis after unilateral ureteral obstruction.
3. The increase in cell death rate in collecting ducts may be one of the underlying mechanisms of renal tubulointerstitial fibrosis development.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2015R1D1A1A09059195) and MRC for Cancer Evolution Research Center (2012R1A5A2047939), and the institute of Clinical Medicine Research of Bucheon St. Mary's Hospital Research Fund, BCMC14IA01.