Medication nonadherence in Korean patients with rheumatoid arthritis: the importance of belief about medication and illness perception
Article information
Abstract
Background/Aims
To investigate medication nonadherence in Korean patients with rheumatoid arthritis (RA) and analyze related factors.
Methods
A total of 292 patients with RA participated in this study. Medication nonadherence, intentional or unintentional, was gauged via self-reported questionnaire. Patient perceptions of illness, treatment beliefs, and moods were measured via Brief Illness Perception Questionnaire, Beliefs about Medicines Questionnaire, and Patient Health Questionnaire-2, respectively. Demographic and clinical data were also collected. Multinomial regression analysis was used to assess the impact of demographic, clinical, and psychological factors on medication nonadherence.
Results
The medication nonadherence rate was 54.1% (intentional, 21.6%; unintentional, 32.5%). Intentional nonadherence was reported most often in patients treated daily drugs (nonsteroidal anti-inflammatory drugs and/or disease-modifying antirheumatic drugs) (24.2%), and unintentional nonadherence was highest in patients receiving methotrexate (33.3%) (p = 0.872). In univariate analysis, beliefs in necessity and concerns of medication differed significantly in adherent and nonadherent patients (intentional or unintentional). When controlling for other factors that may impact medication nonadherence, less belief in necessity of medication (odds ratio [OR], 0.81; 95% confidence interval [CI], 0.68 to 0.95) and greater emotional response to disease (OR, 1.19; 95% CI, 1.01 to 1.40) were important predictors of intentional nonadherence.
Conclusions
Medication nonadherence is common in Korean patients with RA. Less belief in necessity of medication and greater emotional response to disease were identified as key factors prompting intentional nonadherence. These factors may be strategically targeted to improve medication adherence rates and subsequent clinical outcomes.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic systemic and articular inflammatory disease that may result in progressive joint destruction [1]. Nearly 90% of patients with aggressive RA will become clinically disabled within 20 years [2]. Thus, lifetime treatment is generally needed to prevent the joint damage and preserve bone integrity. Disease-modifying antirheumatic drugs (DMARDs), steroids, and nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used to treat RA, and the regimens are often complex [3]. Therapeutic success depends on both drug efficacy and medication adherence. Therefore, medication adherence is essential for desired clinical outcomes [4].
In patients with RA, published adherence rates vary, ranging from 38% to 98.5% [5-13]. These data were generated between 1999 and 2010 from diverse samplings (70 to 14,586 patients) and were largely drawn from Western countries. Still, one study has reported that Hispanic and African-American patients with RA showed less adherence to medications than Caucasian [9], indicating that ethnicity may influence therapeutic compliance. In Korean patients with RA, few studies have addressed medication nonadherence and related factors.
Medication nonadherence may be further characterized as intentional and unintentional on the parts of patients. In the case of intentional nonadherence, patients actively refrain from prescribed regimens. Whereas unintentional nonadherence is the result from others with less intensely held beliefs and perceptions about their medicines [14]. Factors that influence medication nonadherence are important to identify and appreciate, so that appropriate interventions may be formulated. This study was conducted to assess intentional and unintentional medication nonadherence in Korean patients with RA and to determine factors that cause such behaviors.
METHODS
Study populations and design
This prospective cross-sectional investigation was conducted between March 2014 and April 2015. Subjects who fulfilled the American College of Rheumatology diagnostic criteria for RA were recruited from three tertiary hospitals in Korea. A total of 292 adult patients diagnosed with RA for at least 6 months and treated with at least one DMARD qualified for analysis. Each enrollee signed a written informed consent and completed several required questionnaires. The protocol was approved by the Institutional Review Boards of Gyeongsang National University Hospital, Keimyung University Dongsan Medical Center, and Pusan National University Hospital (GNUH 2014-02-009-016).
Data collection
Baseline sociodemographic and clinical characteristics were collected, including age, gender, education level, comorbidities, disease duration, disease activity, and current use of anti-inflammatory and/or antirheumatic medications. Disease activity estimates were based on the Disease Activity Score 28 (DAS28) which is a combined index, incorporating an inflammatory biomarker (erythrocyte sedimentation rate or C-reactive protein [CRP]), physician-rated tenderness and swelling scoring (of 28 joints), and self-reported grading of pain on a 100- mm visual analog scale (VAS). Degrees of depression, illness perception, and medication beliefs were assessed via questionnaires.
Questionnaires
Assessment of adherence
Subjects responded to three questions pertaining to unintentional nonadherence: during the past 2 weeks, (1) did you ever forget to take the prescription medication; (2) did you ever run out of the prescription medication; or (3) when you travel or leave home, do you sometimes forget to bring along your medicine? At least one negative response signaled unintentional nonadherence. Similarly, subjects were questioned on intentional nonadherence: during the past 2 weeks, (1) did you stop taking your medicine without telling your doctor, because you felt worse when you took it; (2) skip taking the medicine because you felt better; or (3) skip doses of medication because of inconvenience? Again, at least one negative response indicated intentional nonadherence. Adherence was considered acceptable if a patient earned a score of 6 on the medication adherence questionnaire. Both sets of adherence measures (unintentional and intentional) were adapted from validated methods published in the peer-review literature [14].
Measurement of depression
Depressive symptoms were assessed using the Patient Health Questionnaire-2 (PHQ-2) [15]. The PHQ-2 contains the first two items of the PHQ-9, which is the full PHQ depression survey. For each item, the response options are “not at all (score = 0),” “several days (score = 1),” “more than half the days (score = 2),” and “nearly every day (score = 3).” Thus, PHQ-2 scores ranged from 0 to 6. At a PHQ-2 cut-point ≥ 3, the best trade-off between sensitivity and specificity both for major depressive disorder and any depressive disorder was evident. The patients were considered depressed at PHQ-2 scores ≥ 3.
Illness perceptions
The Brief Illness Perception Questionnaire (IPQ) [16] was employed to assess patient perceptions of RA. This is an eight-part survey, with each level scored from 0 to 10. There are five cognitive illness representations: consequences (how much does your illness affect your life?); timeline (how long do you think your illness will continue?); personal control (how much control do you feel you have over your illness?); treatment control (how much do you think your treatment can help your illness?); and identity (how much do you experience symptoms from your illness?). Also included are two emotional representations: concern (how concerned are you about your illness?) and emotional responses (how much does your illness affect you emotionally?). Illness comprehensibility (how well do you feel you understand your illness?) is a separate item.
Beliefs about medication
Subjects were further evaluated using the Beliefs about Medicines Questionnaire (BMQ) [17], which has been validated for appraisal of chronic illness groups. The BMQ harbors two five-item scales to assess beliefs that prescribed medication is mandatory for illness control and to explore concerns over potentially adverse treatment effects. Patient responses to specific medicine-related statements are gauged via a five-point Likert scale (1 = strongly disagree to 5 = strongly agree). Scores obtained in each section are summed, generating an overall total in the range of 5 to 25. Higher scores indicate greater belief in prescribed medications.
Statistical analysis
Patient characteristics were analyzed descriptively. The proportion of adherent subjects was calculated according to our definition of adherence. In assessing group differences (adherent vs. nonadherent), paired t tests or chisquare analyses were applied to measured parameters. Multinomial regression analysis was used to test for independent associations between medication nonadherence and the following explanatory variables: gender, age, level of education, disease duration, comorbidities, DAS28- CRP, administration method, depressive symptoms, and grading scales (IPQ and BMQ). All data were analyzed using standard software SPSS version 21.0 (IBM Co., Armonk, NY, USA), setting significance level at p < 0.05.
RESULTS
Patient characteristics
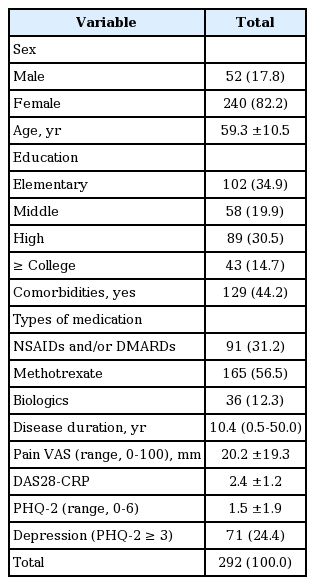
Of the 292 study participants (mean age, 59.3 years), 240 (82.2%) were women and 52 (17.8%) were men. Median disease duration was 10.4 years (range, 0.5 to 50), mean pain VAS was 20.2 mm (standard deviation [SD] ± 19.3), and mean DAS28-CRP was 2.4 ± 1.2. Overall, 91 patients were taking daily NSAIDs and/or DMARDs only, whereas the others received either methotrexate (n = 165) or biologics (n = 36) in addition to daily antirheumatic medicines. Comorbidities were recorded in 129 patients (44.2%). Forty-three participants (14.7%) had at least college-level educations, 89 (30.5%) had high school educations, 58 (19.9%) received middle school diplomas or equivalents at best, and the remaining 102 (34.9%) completed elementary school only. The mean PHQ-2 score was 1.5, and 71 (24.4%) participants had severe depressive moods. Table 1 summarizes baseline patient characteristics.
Medication nonadherence
The overall nonadherence rate was 54.1% (intentional, 21.6%; unintentional, 32.5%). Nonadherence rates in patients on regimens of daily NSAIDs and/or DMARDs, daily NSAIDs and/or DMARDs + weekly methotrexate, and oral antirheumatic drugs (NSAIDs and/or DMARDs) + biologics were 56.0%, 54.5%, and 47.2%, respectively. Intentional nonadherence was reported most often in patients treated with daily NSAIDs and/or DMARDs (24.2%), whereas unintentional nonadherence was highest in patients receiving methotrexate (33.3%). The adherence rate with biologics surpassed those of other oral drugs, but statistical significance was not reached (p = 0.872) (Fig. 1).
Patient differences (adherent vs. nonadherent)
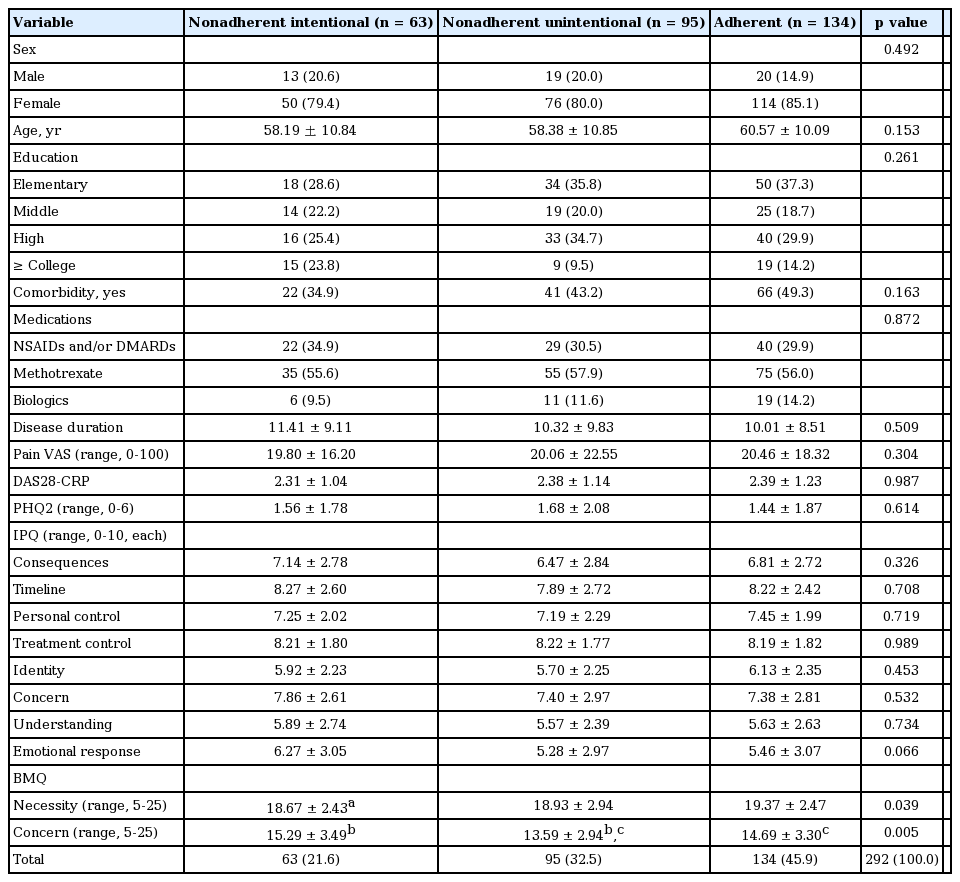
Sociodemographic, clinical, and psychological characteristics of 134 adherent patients were compared with those of 63 intentionally nonadherent patients and 95 unintentionally nonadherent patients (Table 2). There were no significant differences in baseline characteristics between adherents and nonadherents. However, necessity scores in nonadherent patients (intentional, 18.67 ± 2.43; unintentional, 18.93 ± 2.94) were significantly lower than those in adherent patients (19.37 ± 2.47, p = 0.039). In addition, concern scores intentionally nonadherent patients (15.29 ± 3.49) were significantly higher than those recorded in adherent patients (14.69 ± 3.30, p = 0.005). Thus, intentionally nonadherent (vs. adherent) patients conceded to less necessity and more concerns over medications. Unintentionally nonadherent patients were less concerned over medication than were adherent patients.
Determinants of nonadherence (intentional and unintentional)
Multinomial regression was used to identify the strongest predictors of intentional and unintentional nonadherence, given included covariates that may also impact medication adherence (Table 3). When controlling for gender, age, level of education, disease duration, disease activity, comorbidity, medication type, and depressive symptoms, intentional medication nonadherence was associated with a higher emotional response to disease (odds ratio [OR], 1.19; 95% confidence interval [CI], 1.01 to 1.40) and a lower belief in the necessity of medications (OR, 0.81; 95% CI, 0.69 to 0.95). Otherwise, less concern over medication and unintentional nonadherence were significantly related (OR, 0.86; 95% CI, 0.77 to 0.97). Illness perception and beliefs about medication may then serve as important targets in efforts to improve treatment adherence.
DISCUSSION
Our findings indicate that more than half (54.1%) of the patients conceded to medication nonadherence. Within this subset, intentional nonadherence (recorded in 21.6%) showed significant associations with emotional response to disease and the necessity for prescribed medications. In other words, a positive belief in the necessity of medications and a less negative emotional response to RA emerged as strategic factors for improving therapeutic adherence in this setting.
In previous studies, adherence rates in patients with RA vary considerably, ranging from 38% to 98.5% [5-13]. The methods utilized for estimating medication adherence included subjective (patient interviews), direct (chemical markers), and indirect techniques (Medication Event Monitoring System, refill data, and questionnaires). Such rate disparities are therefore attributable to differences in study populations and designs, type of drugs administered, definitions of adherence, and methods of assessment. In this investigation, the medication adherence rate in Korean patients with RA was 45.9%, which is lower than a rate comparably determined by self-reported questionnaire in the United Kingdom [7,13]. However, a Korean study using the same method for assessing adherence to secondary preventive medication in stroke patients reported an adherence rate of 41.2%, similar to results here [14]. Because our subjects with RA were symptomatic, the relatively low rate of adherence that we observed is likely accurate and should fuel efforts for improvement.
We also examined factors associated with medication nonadherence to enable strategic targeting of improvement efforts. Several sources have suggested two broad categories of patient nonadherence: intentional and unintentional. Unintentional nonadherence reflects a questionable capacity for taking medication, including forgetfulness, poor manual dexterity, medication loss, or nonaffordability; intentional nonadherence implies decision-driven behavior, based on patient perceptions of illness and beliefs in prescribed therapies [18-20]. For health professionals, this means that an understanding of pertinent behaviors and types of interventions designed to overcome nonadherence is paramount. We have encountered only one study where intentional and unintentional nonadherence subsets were distinguished in patients with RA [9]. Although the latter combined patients with RA and systemic lupus erythematosus, outcomes were comparable to ours—that is, 20% to 40% of patients admitted to intentional discontinuation of medications. Nevertheless, their reported unintentional nonadherence rate (i.e., two-thirds of patients) exceeded the corresponding rate in this study.
The current investigation also revealed a significant association between beliefs about medication and nonadherence (intentional or unintentional) in patients with RA, corroborating similar evidence from several previous studies. van den Bemt et al. [10] have likewise identified a relationship between belief in the necessity of medications and adherence, but concerns over prescribed medicines did not correlate in tandem. Neame and Hammond [7] also showed that concerns over medication were higher in nonadherent (vs. adherent) patients. However, some earlier studies, unlike ours, have demonstrated that less concern over medication effects and unintentional nonadherence are significantly related. This discord maybe explained by the fact that cognitive functioning was not assessed in the current investigation. Further study is needed to pinpoint why some patients simply forget to take their medications.
At present, few studies have addressed illness perception among patients with RA. Hughes et al. [21] have linked some IPQ domains with medication adherence and one IPQ domain was associated with intentional nonadherence in our patients. Specifically, negative emotional responses to RA encouraged nonadherence to medications. Consequently, the fostering of positive illness perception may improve medication adherence in these circumstances.
The World Health Organization has published an overview of adherence issues, examining ways to improve treatment adherence in a variety of conditions needing long-term therapies [22]. In doing so, they have cited five domains (socioeconomic status, healthcare systems, medical conditions, treatment regimens, and patient-related factors) that contribute to nonadherence. Although we examined the impact of patient age, gender, education level, mental health disorders (such as depression), physical impairments, and disease activity/duration in this study, none were significantly associated with medication nonadherence; additionally, the DAS had no apparent influence on nonadherence, refuting other contentions [11]. Our cross-sectional study design may be culpable in this regard, and the 6-month antirheumatic treatment period required for enrollment may have skewed our results by reducing disease activity. Additional longitudinal studies are needed to better explore the relationship between disease activity and drug adherence.
This study has several acknowledged limitations. A side from its design, which prohibits causality inferences, the potential for selection bias was introduced by recruiting patients from tertiary hospitals and enrolling only adult patients diagnosed with RA for at least 6 months and treated with at least one DMARD. Because of that, the mean disease activity of study population was low. If patients with high disease activity are included, several factors such as disease activity, disease duration, or pain VAS can impact on medication nonadherence. Moreover, the subjective nature of surveys conducted implicates reporting bias as well. Thirdly, 2 weeks might seem to be quite short period to evaluate the adherence in patients with RA. Further study was needed to assess medication adherence during extended period. Finally, other factors that may influence nonadherence, such as patient-doctor relationships, perceived costs, and especially cognitive functions, were not pursued.
In conclusion, our surveys show that drug adherence in Korean patients with RA is a product of greater belief in the necessity of medication and less emotional response to illness. Whenever we treat patients with RA, we can check the BMQ and IPQ to predict adherence. If patients scored low in necessity of medication or high in emotional response to illness, we have to educate the importance of current medication and change patients’ beliefs about medicine positively. And we have to relieve emotional response to illness through education. As such, these aspects of treatment should receive more emphasis to improve adherence rates and subsequent clinical outcomes.
KEY MESSAGE
1. The overall nonadherence rate was 54.1% (intentional, 21.6%; unintentional, 32.5%) in Korean rheumatoid arthritis (RA) patients.
2. Beliefs about medication and illness perceptions were significantly associated with medication nonadherence. Intentional medication nonadherence was associated with a lower belief in the necessity of medications and a higher emotional response to disease.
3. Thus, we have to educate the importance of current medication and relieve emotional response to illness for improving medication adherence in Korean RA patients.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by grants (NRF-2014R1A2A1A11051360 and NRF-2015R1A5A2008833) from the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP).