Computed tomography morphologic features of pulmonary adenocarcinoma with brain/bone metastasis
Article information
Abstract
Background/Aims
Brain and bone metastases are common in patients with lung cancer. The development of metastasis is associated with poor survival in lung cancer patients. Although tumor morphologic features on radiographs are routinely assessed for differentiation between benign and malignant lung nodules, they are not used to predict metastasis. We assessed morphologic features of pulmonary adenocarcinomas with brain/bone metastasis on computed tomography (CT) to identify related factors for metastasis.
Methods
We performed a retrospective analysis of initial chest CT findings (size, type of contour, percentage of necrosis, enhancement, presence or absence of calcification, and air cavity) from 2009 to 2010 of patients with brain or bone metastasis and compared the findings with those of patients without metastases.
Results
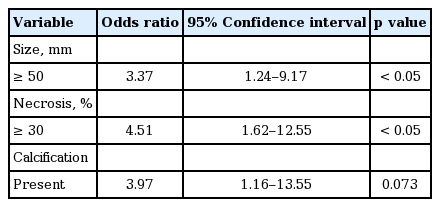
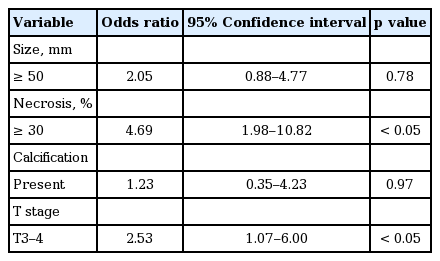
In total, 128 patients were included (78 men, 52 women; mean age 69 years; range, 36 to 87). Nineteen patients had brain metastases and 32 had bone metastases. Morphologic features associated with brain metastasis included size ≥ 50 mm (odds ratio [OR], 3.37; 95% confidence interval [CI], 1.24 to 9.17; p = 0.013), necrosis ≥ 30% (OR, 4.51; 95% CI, 1.62 to 12.55; p =0.002), and presence of calcification (OR, 3.97; 95% CI, 1.16 to 13.55; p = 0.035). Morphologic features associated with bone metastasis included necrosis ≥ 30% (OR, 4.639; 95% CI, 1.98 to 10.82; p < 0.001) and T 3 to 4 stage (OR, 2.53; 95% CI, 1.07 to 6.00; p = 0.031).
Conclusions
We found that necrosis ≥ 30% was associated with pulmonary adenocarcinoma with brain and bone metastasis at initial chest CT morphologic feature. To validate these results, further research should be conducted.
INTRODUCTION
The metastatic spread of cancer to distant organs is the reason for most cancer deaths [1-4]. Lung cancer is the most frequent and among the most lethal types of cancer worldwide. Approximately 50% of lung cancer cases are metastatic at diagnosis [5]. The major sites of non-small cell lung cancer (NSCLC) metastases include the brain (47%), bone (36%), liver (22%), adrenal glands (15%), thoracic cavity (11%), and distant lymph nodes (10%) [6].
Although morphologic features of tumors on radio graphs are routinely assessed for differentiation between benign and malignant lung nodules, they are not used to predict lung cancer metastasis. There are two significant groups of predictive factors for metastasis: TNM-related factors and tumor-related factors. The TNM-related group comprises information related to tumor size, lymph node, and metastasis factors [7,8], while the tumor-related group comprises histopathological, anatomical, and biochemical factors [9]. On the other hand, morphologic features of tumors that are easily assessed by computed tomography (CT) have not yet been investigated as potential predictive factors for the development of metastasis. In lung cancer, chest CT is routinely used for patient management, including diagnosis, radiation treatment planning, and response evaluation after treatment. In addition, CT is used to evaluate tumor size, lymph node invasion, and metastasis.
Some studies focusing on imaging modalities have suggested that radiologic findings could be a predictive factor for metastasis. However, many of these studies were conducted in patients with resectable early stage lung cancer, so the result cannot be representative of all patients with lung cancer. Among lung cancer, pulmonary adenocarcinomas grow more slowly and form smaller masses than the other subtypes. However, they tend to form metastases widely at an early stage [10]. Thus, we assessed the CT morphologic features of pulmonary adenocarcinomas with brain/bone metastasis with the goal of identifying related factors for metastasis.
METHODS
We retrospectively reviewed the medical records of 256 patients with lung cancer between January 2009 and December 2010. Only patients with a pathologically confirmed diagnosis of primary pulmonary adenocarcinoma and available CT scans in the Picture Archiving and Communication System were included. Patients were diagnosed with brain metastasis on brain CT or magnetic resonance imaging and bone metastasis on bone scan or positron emission tomography-CT at the time of the initial diagnosis of lung cancer. Ultimately, 128 patients were included in the study. In this study, patients with adrenal gland, liver metastasis were excluded for fewer cases.
We collected clinical data such as age, sex, smoking history, lung cancer staging, and histological type, and CT morphologic features such as size, type of contour, percentage of necrosis, enhancement, presence or absence of calcification, and air cavity.
Two radiologists analyzed the patients’ CT morphologic features as if making the initial diagnosis. They did not have any of the patients’ data while evaluating the initial chest CT images. Imaging features that are common in lung cancer patients were used as items of CT morphologic findings for subgroup analysis. By comparing pre- and post-enhancement images without delay, we determined a standard site width difference between the enhancement of identical site lesions. With this standard, we defined necrosis as a site of decreased Hounsfield units, similar to the process of fat deposition. Additionally, we separated the findings into two different groups: one with more than 30% of the lesion occupied by necrosis, and the other with less than 30% occupied (Fig. 1). Calcification was defined when the CT findings had similarities to bone density. The definition of an air cavity was a gas-filled or air density space within a pulmonary lesion, such as a consolidation, mass, or nodule, and these findings were qualified. Air cavities included air bronchograms and small air spaces in lesions. The type of contour was divided into round, speculated, and linear. TNM staging was performed using the American Joint Committee on Cancer Cancer Staging Manual, 7th edition.
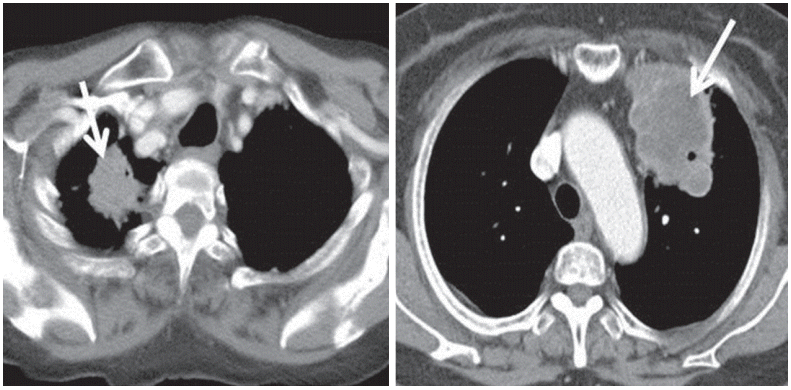
Illustration of computed tomography features of pulmonary adenocarcinoma investigated in this study. (A) Necrosis (–), (B) necrosis (+). Arrow indicates features of interest in each image.
Statistical analysis of the relationship between each of the variables and the presence of metastasis was performed using the chi-square test or Fisher exact test. The odds ratio was calculated from the frequency of each of the variables compared between metastasis-positive and metastasis-negative patients. Univariate analysis was first applied to assess the association between each of the variables and mortality. Variables selected by univariate analysis (p < 0.05) and those considered as clinically relevant were entered in a logistic regression model. The Kaplan-Meier method and log-rank test were used to perform univariate survival analysis. All statistical analyses were performed with SPSS version 18 (SPSS Inc., Chicago, IL, USA). A value of p < 0.05 was considered statistically significant.
RESULTS
Patients
Materials and data from 78 men (60%) and 52 women (40%) were included in the study. The patients ranged in age from 36 to 87 years (median, 69.0). Among the 128 included patients, 60 patients had stage 4 disease, 46 patients had stage 3, four patients had stage 2, and 18 patients had stage 1 disease.
Table 1 shows the CT morphologic features of the tumors. The median diameter of all measured tumors was 44.24 mm (range, 10 to 176).
CT morphologic features in brain metastasis group
Brain metastasis was identified in 19 patients. Table 2 provides information on patient demographics and CT findings at the time of initial diagnosis in the brain metastasis group. By univariate analysis, the brain metastasis group was found to have a significantly higher number of patients with the following characteristics at the time of diagnosis: size ≥ 50 mm (37.0% vs. 10.9% for < 50 mm, p = 0.013), necrosis ≥ 30% (40.0% vs. 5.0% for < 30%, p = 0.002), and presence of calcification (50.0% vs. 14.0% for absence, p = 0.035).
CT morphologic features in bone metastasis group
Bone metastasis was identified in 32 patients. Table 3 shows the clinical characteristics and imaging features obtained on chest CT of the patients in the bone metastasis group. By univariate analysis, the bone metastasis group was found to have a significantly higher number of patients with the following characteristics at the time of initial diagnosis: necrosis ≥ 30% (82.6% vs. 17.8% for < 30%, p < 0.001), and T stage 3 to 4 (48.7 % vs. 19.2 % for stage 1 to 2, p = 0.031).
Multivariate analysis
On the basis of the results of univariate analysis, each of the statistically significant variables was subjected to stepwise multivariate analysis. Tables 4 and 5 show the results of multivariate analysis. We identified that necrosis ≥ 30% was a common related factor for brain and bone metastasis as an initial chest CT morphologic feature. There was no statistically significant correlation of necrosis to patient’s survival (p = 0.20).
DISCUSSION
Lung cancer frequently metastasizes to bone, brain, lung, and liver, resulting in shorter survival [11]. Therefore, increased knowledge of metastasis is crucial in the treatment of patients. Previous studies revealed that morphologic features of tumors on radiographs are routinely assessed for differentiation between benign and malignant lung nodules. In the current study, we assessed CT morphologic features of pulmonary adenocarcinomas with brain/bone metastasis to identify predictive factors for metastasis. We found that the features observed on chest CT most significantly associated with an increased risk of brain metastasis were size ≥ 50 mm, necrosis ≥ 30%, and calcification, and those associated with an increased risk of bone metastasis were necrosis ≥ 30% and T stage 3 to 4. A finding of necrosis ≥ 30% on chest CT was a common related factor for the development of brain/bone metastasis.
Various studies have proposed a close correspondence between CT morphologic features and prognosis. Takashima et al. [12] demonstrated that the percentage of ground glass opacity (GGO) area measured by high-resolution CT was a significant factor for the survival of lung cancer patients. Aoki et al. [13] showed that the frequencies of lymph node metastasis and vessel invasion in adenocarcinomas with GGO components of more than 50% were significantly lower than those in adenocarcinomas with GGO components of less than 10% obtained by thin-section CT findings. There have also been studies focusing on the relationship between CT morphologic characteristics as well as tumor size and TNM grade [14-17]. Bajard et al. [18] showed that clinical T classification was significant for the development of brain metastasis in a cohort of 305 NSCLC patients. In the current study, T stage was associated with the incidence of bone metastasis. Even though nodal involvement is part of TNM staging, we were unable to find any relationship between CT morphologic features and metastasis in this study.
Some studies, researching prognostic factors rather than predictive factors, found that tumor necrosis was associated with poor outcomes in lung cancer patients. Inoue et al. [19] reported that microscopic necrosis was associated with tumor recurrence, leading to decreased 5-year disease-free survival rates. In another study by Kilicgun et al. [20] and Park et al. [21], the authors reported that tumor necrosis was a significant adverse risk factor for cancer recurrence and was also associated with patients’ overall survival [22,23]. The majority of results from those studies have statistical significance in terms of prognostic value or patient survival rates, but they are limited to patients with early stage lung cancer and have little to offer regarding predictive value.
Necrosis is associated with an unusual, passive, and unregulated pathway of cell death caused by injury, damage, and ischemia [24]. Death of cells resulting from extreme conditions such as temperature change or inflammatory reaction causes an unregulated process of destruction of cell membranes and the cytosol, and finally cell digestion. Particularly in the case of tumor cell necrosis, a pattern of coagulative change is the most common. This is caused by tissue hypoxemia due to rapid tumor cell growth, incomplete neovascularization, and hypoperfusion of adjacent vessels [25-27]. By this mechanism, tumor necrosis measured by CT is the result of a time-dependent element and could play a role as a predictive factor associated with cancer metastasis.
In our study, the presence of an air cavity did not have statistical significance. However, some studies have reported that an air cavity has occurred following tissue necrotic change. A cavity has been defined as a gas-filled space within a pulmonary nodule, mass, or consolidation. It is produced by the expulsion of a necrotic part of a lesion. By this mechanism, necrosis and cavities may have a correlated time-dependent relationship [28]. Some studies have shown that the presence of an air cavity with lung cancer tissue was associated with a greater likelihood of progression to metastasis and recurrence compared to the absence of an air cavity lesion, so patients with an air cavity had poorer overall survival. Although the mechanism of cavity formation is often difficult to ascertain, cavitation in lung cancer most often results from rapid tumor growth that exceeds the blood supply, with the result of central necrosis [29-31]. Therefore, we expect that the presence of cavitation assessed by CT may be another predictive factor for the development of lung cancer metastasis, and the question should be further investigated through prospective studies with large numbers of patients and long-term follow-up.
The present study has several limitations, including the retrospective observational design, leading to the possibility that selection bias influenced the significance of our findings. Our study is also from a single institution and has a small sample size, factors which limit the generalizability of our findings to other institutions. In addition, evaluation of the percentage of necrosis would involve artifactual variations between pre- and post-enhancement in relation to a modality quality factor. Further research, using computer aided medical image diagnosis system, should be conducted to assess additional related factors for metastasis and survival in lung cancer.
In conclusion, features observed on chest CT most significantly associated with an increased risk of brain metastasis were size ≥ 50 mm, necrosis ≥ 30%, and calcification, and those associated with an increased risk of bone metastasis were necrosis ≥ 30% and T stage 3 to 4. A finding of necrosis ≥ 30% on chest CT was a common related factor for the development of brain/bone metastasis. To validate these results, further research should be conducted.
KEY MESSAGE
1. In this study, we assessed computed tomography (CT) morphologic features of pulmonary adenocarcinomas with brain/bone metastasis to identify predictive factors for metastasis. A finding of necrosis ≥ 30% on chest CT was a common related factor for the development of brain/bone metastasis.
2. Further research, using computer aided medical image diagnosis system, should be conducted to assess additional related factors for metastasis and survival in lung cancer.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (H12CO110), and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI15C1951).