Initial titration with 200 μg fentanyl buccal tablets: a retrospective safety analysis in Korean cancer patients
Article information
Abstract
Background/Aims
Managing breakthrough pain (BTP) is important for many cancer patients because of the rapid onset and unpredictable nature of the pain episodes. Fentanyl buccal tablets (FBTs) are a rapid-onset opioid indicated for BTP management. However, FBT titration is needed to optimize BTP management. In this study, we aimed to evaluate the safety and efficacy of initiating 200 μg FBTs in Korean cancer patients.
Methods
A retrospective analysis of medical records was performed on all advanced cancer patients treated with FBTs for BTP between October 2014 and July 2015. Patients who received initial doses of 200 μg FBTs for at least 3 days and cases in which FBT was available at doses of 200, 400, and 800 μg were included.
Results
A total of 56 patients with a median age of 62 years (range, 32 to 80) were analyzed, 61% of whom were male. The median and mean values of morphine equivalent daily doses were 60 mg/day (range, 15 to 540) and 114.8 ± 124.8 mg/day, respectively. The most frequent effective doses of FBT were 200 μg (41 patients, 74%) and 400 μg (12 patients, 21%). Three patients (5%) could not tolerate 200 μg of FBT and discontinued treatment. Nausea, vomiting, somnolence, and dizziness were the most frequent treatment-related adverse events (AEs), and all AEs were grade 1 (mild) or 2 (moderate).
Conclusions
FBT at the initial 200 μg dosage was well-tolerated and effective as a BTP management strategy in Korean cancer patients. Further prospective studies are needed to determine appropriate initiating doses of FBT in Korean patients with opioid tolerance.
INTRODUCTION
Pain is a major factor that can impair the quality of life in patients with advanced cancer [1]. About 75% of cancer patients experience transitory exacerbation of pain despite appropriate treatment with around-the-clock (ATC) opioids [2]. This breakthrough pain (BTP) is also a serious problem for advanced cancer patients because of the rapid onset and often unpredictable nature in pain episodes. Although BTP is a specific condition for individual patients, BTP is generally defined as a transitory exacerbated pain that occurs over and above controlled background pain [2]. The duration is usually brief and reaches peak intensity within 10 to 15 minutes [3,4]. BTP is associated with adverse effects on patient mood and function [5]. Because of their onset time and duration, oral opioids are considered unsuitable for treating BTP events; therefore, several analyses have suggested that fentanyl formulations are a more efficacious treatment option than oral morphine [6,7].
Fentanyl buccal tablet (FBT; Fentanyl citrate, Fentora, Teva-Handok, Seoul, Korea) is a rapid-onset opioid (ROO) indicated for managing BTP in cancer patients with opioid tolerance [8]. Transmucosal delivery of fentanyl leads to rapid absorption across the buccal mucosa [9]. Treatment with FBT can significantly relieve pain within as few as 10 minutes after administration, resulting in an improved patient satisfaction [10]. For BTP management, a rescue dose of a short-acting opioid (SAO) is generally recommended 10% to 20% of 24-hour ATC opioids dose; however, when using FBTs, a dose titration process starting at the lowest dose, such as 100 μg, has been commonly recommended to provide effective pain control while minimizing the risk of clinically significant adverse effects [10-12]. The titration process of FBTs was attributed to the finding that the effective dose of transmucosal fentanyl opioids could not be predicted from the maintenance dose of the ATC opioids [13]. Interindividual variations in opioid metabolism and response to opioids have also been suggested to affect the efficacy of these drugs [10].
However, a straightforward dose titration process using lowest starting dose can be time consuming for determining the appropriate FBT dose and may result in poor compliance [10]. Thus, a faster and simplified management of BTP is needed. The initial FBT dose also remains controversial similar to the optimal starting doses of transdermal fentanyl for chronic cancer pain [14]. Therefore, several Western studies evaluated the initial FBT doses proportional to the high doses of ATC opioids used for background pain and determined that it was effective and well tolerated [15-17]. In addition, a recent randomized study conducted in Europe compared a starting dose of FBTs at 100 or 200 μg in the titration process, and established non-inferiority in terms of achieving an effective dose by starting with 200 μg of FBTs (81.4%) compared with 100 μg (75.2%) [18]. Adverse events (AEs) during the titration period were also similar between the two groups (5.5% in 100 μg group, 7.2% in 200 μg). Taken together, these results suggested that a starting titration at 200 μg of FBTs is a possible strategy in European clinical practice.
Asian cancer patients differ from Western patients in terms of body weight and body mass index (BMI) [19]. It was not known whether initiating titration of FBTs at a dose of 200 μg will be safe and feasible in Asian patients. Additionally, in clinical practice settings, 100 μg FBTs was not available in about 20% of hospitals in Korea, including at our institution. Therefore, we aimed to evaluate the safety and efficacy of initiating 200 μg FBT for BTP in Korean cancer patients.
METHODS
Patients and data collection
We retrospectively reviewed the medical records of 136 consecutive patients with advanced cancer that were treated with FBT for BTP between October 2014 and July 2015 at Kangbuk Samsung Hospital (Seoul, Korea) where the FBT was available at doses of 200, 400, and 800 μg. Patients were eligible for this study if they were 19 years of age or older, with histologically documented malignancy, if they received an initial FBT of 200 μg for at least 3 days, and if they were able to evaluate their pain intensity and AEs according to medical records. This study was approved by the Institutional Review Board of Kangbuk Samsung Hospital.
BMI is calculated as body weight (kg) divided by the square of body height (m). The BMI categories are as follows: less than 17.5 kg/m2 is very underweight; 17.5 to 18.4 kg/m2 is underweight; 18.5 to 22.9 kg/m2 is normal; 23 to 24.9 kg/m2 is overweight; and 25.0 kg/m2 or higher is obese [20]. Background pain intensity was assessed as average day pain intensity before administering FBT using the Numeric Rating Scale (NRS-11) with scores that ranged from 0 (no pain) to 10 (pain as bad as you can imagine). The final effective dose was assessed from medical records where physicians’ assessments of successful pain relief and AEs for the FBT dosage administered. For dose titration of FBT, the dose was continuously increased, to 400 and 800 μg, for subsequent BTP episodes when BTP was considered to be unsatisfactory controlled to achieve an effective dose. Safety was assessed by reported AEs, which were evaluated using Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Statistical analyses
Demographic and clinical variables were collected, and descriptive statistical analysis of relevant variables was performed to obtain frequency distributions, mean ± standard deviations. Baseline ATC opioids were converted to oral morphine equivalent daily doses (MEDDs), and MEDD were compared according to the final doses of FBT using analysis of variance. Associations between AEs and opioid-naïve or tolerant status were assessed by chi-square analysis or Fisher exact test. A two-sided p < 0.05 was considered significant, and 95% confidence intervals were calculated. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
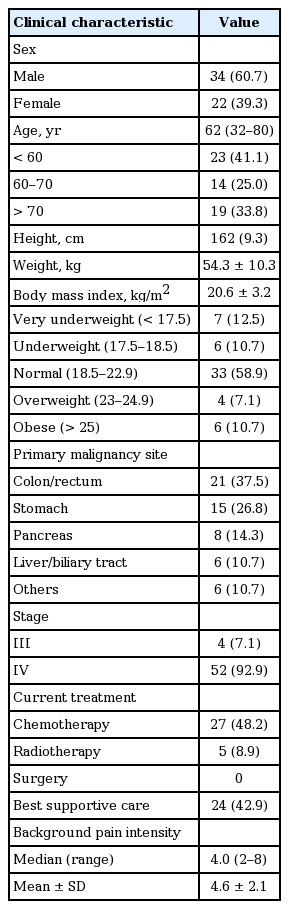
A total of 56 patients were eligible for this study. Baseline characteristics of patients at initiation of 200 μg FBT are summarized in Table 1. Males represented 61% of the patients, and the median age was 62 years. Mean body weight was 54.3 kg, and mean BMI was 20.6 kg/m2. Patients classified in the very underweight or underweight categories represented 24% of all patients. The most common malignancy originated from colorectal cancer (38%), and 93% of all cases were stage IV. The median background pain intensity was 4.0 (range, 2 to 8), and the mean value was 4.6 ± 2.1.
Baseline around-the-clock opioids and short-acting opioids
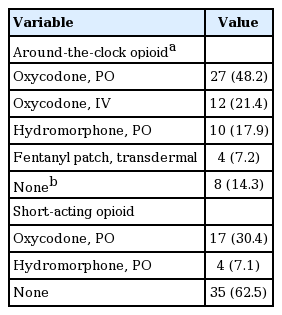
The types of baseline ATC opioids and SAOs are summarized in Table 2. A total of 48 patients (86%) were treated with ATC opioids. The median and mean values of oral MEDD were 60 mg/day (range, 15 to 540) and 114.8 ± 124.8 mg/day, respectively, and 31 patients (55%) received 60 mg/day or more of oral MEDD. Among the eight patients who had not received ATC opioid, only one patient had already used SAO (oxycodone 60 mg/day), and the other seven patients (12%) were opioid-naïve.
Final effective doses of fentanyl buccal tablet
Overall, the most frequent effective doses of FBT were 200 μg (41 patients, 74%), and 400 μg (12 patients, 21%). The most frequent numbers of daily uses of FBT were 3 to 4 times/day (32 patients, 58%), 1 to 2 times/day (18 patients, 32%), and 5 or more times/day (three patients, 5%). According to the final FBT dose, there was an increasing trend of MEDD, although this result was not statistically significant (p = 0.227), even when the seven opioid-naïve patients were excluded (p = 0.321) (Table 3, Fig. 1A). Although a statistically significant difference in MEDD was observed according to the number of daily uses of FBT (p = 0.036), even after excluding the seven opioid-naïve patients (p = 0.028) (Fig. 1B), this statistical difference did not seem clinically significant because of the small number of participants and lower MEDD in the patient group that used FBT 5 or more times/day (Table 3).
FBT-related AEs and intolerance of 200 μg FBT
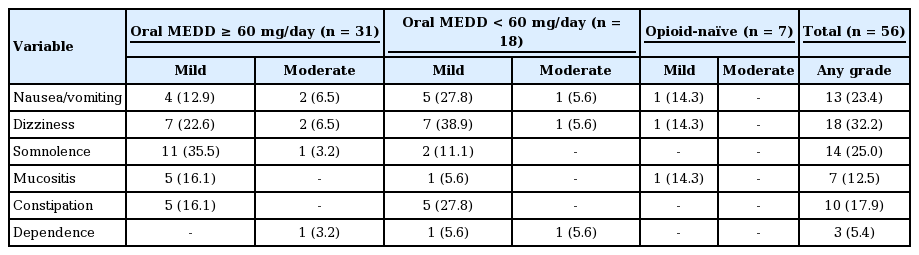
Our results indicated that 38 patients (68 %) experienced at least one treatment-related AE over a median 8.3 months of follow-up (range, 1.2 to 10.9). Nausea/vomiting, somnolence, and dizziness were the most frequent treatment-related AEs. All AEs were grade 1 (mild) or 2 (moderate), and no significant difference in AE incidence was observed according to sex, age, or BMI. When the incidence of AEs was compared according to oral MEDD (opioid-naïve, oral MEDD < 60 mg/day, or ≥ 60 mg/day), there was no statistically significant difference (Table 4). However, the incidence rates of nausea/vomiting, dizziness, constipation, and dependence were slightly higher in patients who received < 60 mg/day of oral MEDD compared to those who received ≥ 60 mg/day. There were three patients (5%) who could not tolerate the initial 200-μg FBT dosage and discontinued FBT treatment. The AEs that led to discontinuation of FBT were dizziness in all three patients (100%) and nausea/vomiting in two patients (67%). Although there was no statistically significant factor associated with intolerance to 200 μg FBT, all three of these patients were female and all three had the following clinical features: relatively advanced age (71, 69, and 62 years), low BMI (14.7, 17.6, and 20.2), and low oral MEDD (30, 60, and 60 mg/day).
DISCUSSION
This study was a retrospective analysis of the safety and efficacy of using an initial dose of 200 μg FBTs in Korean cancer patients. Most patients well-tolerated the initial exposure to 200 μg FBT, and, for most, the final effective dose was 200 μg. Dizziness and nausea/vomiting were the most common causes of FBT discontinuation.
Many studies have shown that FBT is effective for managing BTP and is generally well-tolerated in cancer patients with opioid tolerance [13,21,22]. Currently, management of cancer pain guidelines suggest considering rapidly acting transmucosal fentanyl in opioid-tolerant patients for BTP, and always initiating transmucosal fentanyl with lowest dose in chosen formulation and titrate to effect [12]. Although individualized treatment is important, the dose titration process from lowest dose to determine the effective dose in each patient may complicate the practical use of FBT, particularly in outpatient settings [16]. Patients already receiving high doses of opioids for background pain could have trouble managing BTP with dose titration using minimal initial doses of FBT because they are opioid-tolerant [5]. Trying different doses of FBT for each BTP episode may be time-consuming due to the repeated need for dose calculation and adjustments [11,16]. Patients may also be unwilling to attempt the titration process and avoid using the ROOs such as FBT, ultimately preferring traditional oral opioids such as SAOs [23]. Therefore, several studies have been performed to facilitate effective control of BTP using FBT. A European study reported that initiating a titration of FBTs at a 200-μg dose may lead patients to be more rapidly titrated to an effective dose compared with starting at a 100-μg dose [18]. Another multicenter prospective randomized study also indicated that doses proportional to the basal opioids for background pain might be safe and effective, and there is no evidence for the use of FBT dose titration [17].
In the present analysis, 200 μg was the most common dose for the final effective regimen of FBT. Although data from Western clinical studies of FBT show that the effective dose ranged from 100 to 800 μg per episode [13,21,24], the final effective doses of FBT for Asian patients, including our data, are slightly lower than those for Western patients [25]. This could be because Asian cancer patients have lower BMI (20 to 21) and body weights (54 to 59 kg) than Western patients (BMI, 24 to 27; weight, 70 to 80 kg) [13,18,22,25,26] or because clearance of fentanyl differs between lean and obese patients [27].
The most frequent treatment-related AEs were nausea/vomiting, somnolence, and dizziness. The prevalence of these AEs was comparable to previous Western reports [18,22]. About 5% of patients could not tolerate the 200-μg dose, which was also similar to the results of a previous European study [18]. Although factors that predicted intolerance of the initial 200 μg FBTs could not be defined, some clinical features in common for the patients were female sex, advanced age, low BMI (or body weight), and low MEDD. According to pharmacogenomics data, it is known that the μ-opioid receptors are more sensitive in females, so women usually require lower dose of opioids for pain relief and can suffer more from the AEs of opioids compared with male [28]. Another important clinical factor that can affect drug tolerance is patient age. There are changes in body composition with aging: an increase in adipose tissue, decrease in lean body mass and decrease in total body water. These changes can affect drug distribution, and lipophilic drugs such as fentanyl could have a greater volume of distribution and take more time to be eliminated from the body [29]. The elderly also have limitations in physical and functional capacity, so there were considerable interindividual heterogeneities in response to drugs [30]. Therefore, attention is needed when FBT titration is carried out in cancer patients who are female, elderly, underweight, and/or have lower MEDD.
In this study, some opioid-naïve patients were administered FBTs although they are currently indicated only for opioid-tolerant patients [8]. All patients were given FBTs in an inpatient setting by resident physicians in the oncology ward. Fortunately, there were no severe or critical AEs, as fentanyl-related deaths happen not infrequently [31]. Therefore, more education about cancer pain management should be emphasized, especially for non-oncology doctors.
Our study had several limitations that are inherent to the retrospective design and analysis. There were a small number of patients, and all were treated at a single institution. Not all patients were opioid tolerant, which was defined as receiving > 60 mg/day of oral morphine, > 30 mg/day of oral oxycodone, > 8 mg/day of oral hydromorphone, and > 25 mg/hour of transdermal fentanyl or an equivalent dose of opioid [12]. Not all patients had stable and adequately controlled background pain, which was defined as an average pain intensity score < 4/10 on the NRS [32]. Data regarding BTP intensity at and after initial titration with FBTs were not available, and supplemental medication information was not provided. Despite these limitations, this analysis provides practical information on the prevalence of AEs and tolerance to an initial 200-μg dose of FBTs in Korean patients.
In conclusion, an initial dose of 200 μg FBTs was well-tolerated in a clinical practice setting in Korean patients. Further prospective studies are needed to determine the appropriate initiating doses of FBT in Korean cancer patients to develop a safe and effective titration strategy for BTP.
KEY MESSAGE
1. Fentanyl buccal tablets at an initial 200-μg dosage was well-tolerated and effective for break-through pain management in Korean cancer patients.
2. Further prospective studies are needed to determine the appropriate initiation dose in Korean patients with opioid tolerance.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported in part by a Samsung Biomedical Research Institute grant and by Medical Research Funds from Kangbuk Samsung Hospital.