Nontuberculous mycobacterial infection in rheumatoid arthritis patients: a single-center experience in South Korea
Article information
Abstract
Background/Aims
Nontuberculous mycobacteria (NTM) infection has been increasing worldwide in both general population and immunocompromised patients, which has also been reported in rheumatoid arthritis (RA) patients. This study aimed to identify the incidence and clinical characteristics of NTM infection in RA patients living in tuberculosis (TB) infection endemic area.
Methods
We performed a retrospective analysis of NTM infection cases in our RA registry at a tertiary referral center from January 1995 to December 2013. The clinical features of them were compared to those of 52 TB infection patients from same registry.
Results
Among 1,397 patients with RA, NTM infection was newly developed in 26 patients and the incidence of NTM infection was 164.8 per 100,000 patient-years. The Mycobacterium avium complex was the most frequent isolate (76.9%). None of the NTM infections had extrapulmonary involvement, which was rather common in TB infection (26.9%). Patients with NTM infection were older, received higher cumulative steroid doses, and had higher rates of past TB infection history and concomitant interstitial lung disease (ILD) than cases with TB infection.
Conclusions
In South Korea, NTM infection is not rare in RA patients, and infection rates are growing. Physicians should be cautious about NTM infection in patients with a history of TB infection or concomitant ILD, even living in TB endemic area.
INTRODUCTION
Nontuberculous mycobacteria (NTM) are a family of the Mycobacterium species distinct from the Mycobacterium tuberculosis complex and Mycobacterium leprae [1]. They are ubiquitous organisms in the environment and can reside in soil, natural water, or tap water [2]. Previously, NTM was not thought to be a common disease pathogen in immunocompetent hosts, and NTM infections have been seen more frequently in immunocompromised patients, such as organ transplant recipients, cancer patients, and patients with acquired immunodeficiency syndromes [3,4].
Patients with rheumatoid arthritis (RA) are thought to have an increased risk of developing infections, which may be related to immunologic disturbances caused by RA itself or to immunosuppressive therapies [5-7]. To date, there have been several studies on NTM infection in RA patients [8-13]. However, most of these studies focused on NTM infection during anti-tumor necrosis factor (TNF) therapy or were conducted in Western countries where NTM infection is more common than tuberculosis (TB) infection.
In our current study, we describe the incidence and clinical characteristics of NTM infection in RA patients by comparison with TB infection, which is endemic in South Korea.
METHODS
A retrospective, longitudinal cohort study was performed on RA patients at a tertiary hospital in South Korea. From January 1995 to December 2013, a total of 1,428 patients were diagnosed with RA. Of these, 31 were excluded because of a short follow-up duration (< 6 months). Therefore, electronic medical records from 1,397 patients were reviewed. All data available up to February 28, 2015 were used. A diagnosis of NTM infection was made using the criteria proposed by the American Thoracic Society/Infectious Disease Society of America (ATS/IDSA) in 2007 [14]. TB was diagnosed if M. tuberculosis was isolated from any clinical specimen or if DNA for M. tuberculosis was detected by polymerase chain reaction analysis in any clinical specimen (bacteriologically confirmed TB) [15]. Patients with negative mycobacterial culture results but a high clinical likelihood of active TB infection and good clinical/radiographic responses to anti-TB treatment were also considered active TB patients (clinical TB infection). Clinical information was collected at the time of diagnosis of NTM or TB infection in the following areas: demographic characteristics, laboratory data, radiologic findings, microbiological data, and treatment outcome. Radiological abnormalities were classified according to the following four patterns seen on chest computed tomography: nodular/bronchiectatic (NB), fibrocavitary (FC), NB + FC, and other types [16].
Statistical analysis
All continuous variables were expressed as mean ± SD. Categorical variables were reported as numbers and percentages. To estimate the incidence of NTM and TB infection among RA patients, we divided the number of NTM or TB cases by the total number of patient-years of follow-up. Continuous variables were compared by a Student t test or the Mann-Whitney U test, while categorical variables were compared by the chi-square test or Fisher exact test. Statistical significance was defined as p < 0.05. Data were analyzed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
This study fulfilled the ethical guidelines of the Declaration of Helsinki (as revised in Brazil 2013) and received approval from the Institutional Review Board of the Asan Medical Center (number 2015-0333).
RESULTS
Incidence of NTM infection
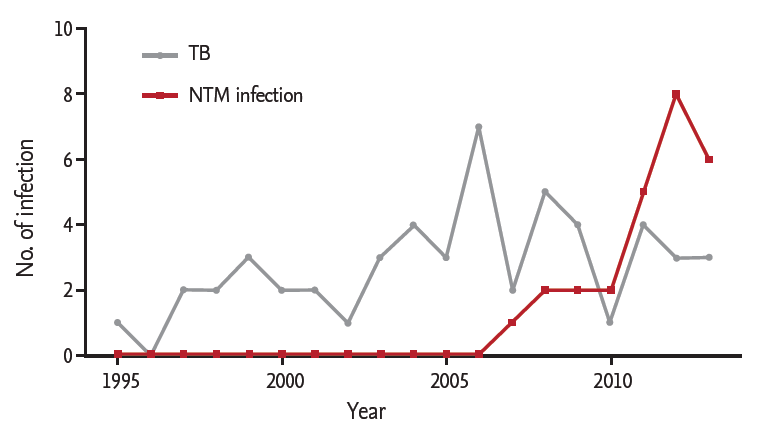
Among 1,397 RA patients, 26 cases of NTM infection and 52 cases of TB infection occurred during the follow-up period (11.1 ± 6.7 years). NTM was isolated from the clinical specimens from 111 patients, but only 26 patients (23.4%) satisfied the diagnostic criteria for NTM infection [14]. The total patient-observation duration of RA patients was 15,780 patient-years, and the incidence of NTM and TB infection after RA diagnosis was 164.8 per 100,000 patient-years and 329.5 per 100,000 patient-years, respectively. NTM infections were increased annually, whereas TB infection has developed in a relatively steady pattern (Fig. 1).
Baseline characteristics of patients with NTM and TB infection
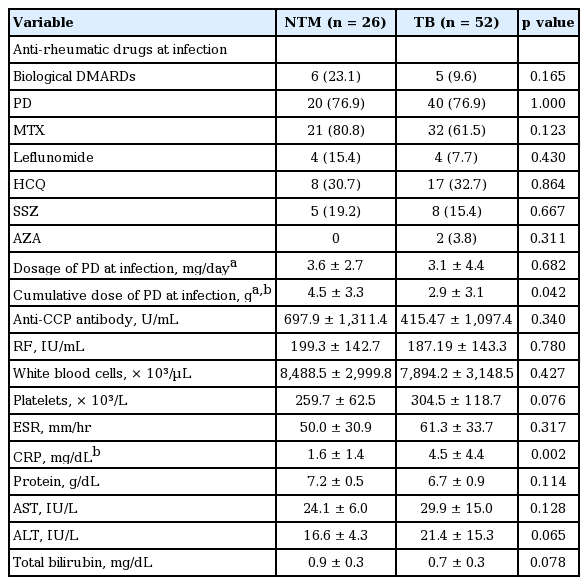
The baseline characteristics of patients with NTM and TB infection are presented in Table 1. The majority of both groups were found in female patients. The age of diagnosis was greater for NTM infections than TB infection, although age of RA diagnosis did not differ between infection types. The average body mass index was below 21 kg/m2 in both groups. A past history of TB infection or concomitant interstitial lung disease (ILD) was more common in patients with NTM infection than cases with TB infection.
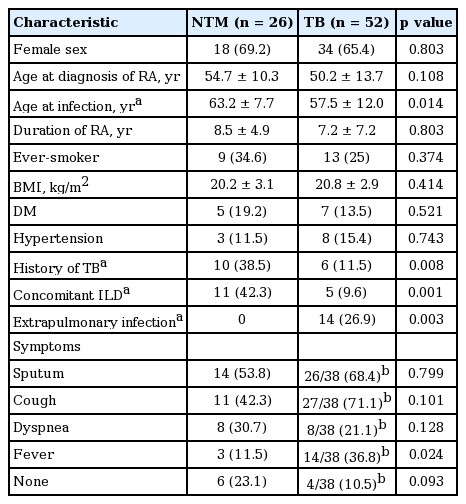
Clinical characteristic of the study patients with nontuberculous mycobacteria infection and tuberculosis
The most frequent symptom of NTM infection in RA patients was sputum. Fever was noted in only three of the 26 NTM patients (11.5%) but in 14 of the 38 pulmonary TB patients (36.8%). All 26 NTM patients showed only pulmonary NTM infection, whilst 14 of the TB patients (26.9%) showed extrapulmonary infections (pleurisy [n = 5], lymphadenitis [n = 3], peritonitis [n = 2], military TB [n = 1], mastitis [n = 1], enteritis [n = 1], and renal TB [n = 1]). Among 26 NTM patients, all patients had a positive test in sputum culture, 15 patients (57.7%) in bronchoalveolar lavage specimen and two patients (10%) in biopsy specimen, respectively.
Medication and laboratory findings for NTM infections
Among the 26 patients in our current series with NTM infection, six (23.1%) were treated with biological disease modifying anti-rheumatic drugs (DMARDs) at the time of diagnosis (etanercept [n = 4], infliximab [n = 2]). For TB patients, five cases (9.6%) were treated with biological DMARDs (abatacept [n = 3], etanercept [n = 1], infliximab [n = 1]) (Table 2). The cumulative steroid dose at the time of diagnosis was 4.5 ± 3.3 g (equivalent dose with prednisolone) in the NTM group, which is higher than the TB patients. However, there was no difference in daily steroid dosage between the two groups at the time of diagnosis. There were no differences in the levels of anti-cyclic citrullinated antibody, rheumatoid factor, white blood cell count, platelet count, erythrocyte sedimentation rate (ESR), protein, alanine transaminase, aspartate transaminase, or total bilirubin between the two groups, with the exception of hemoglobin and C-reactive protein (CRP).
Microbiological and radiological features of NTM infection
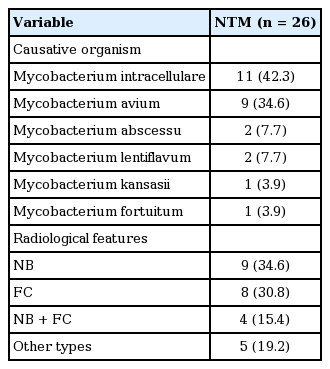
The most common species of NTM infection in RA patients was the Mycobacterium avium complex (MAC) (76.5%; Mycobacterium intracellulare [n = 11], M. avium [n = 9]), followed by Mycobacterium abscessus (n = 2) and Mycobacterium lentiflavum (n = 2) (Table 3). Radiologic features indicated NB type in nine patients (34.6%), FC type in eight patients (30.8%), NB + FC type in four patients (15.4%), and other types in five patients (19.2%).
Treatment and outcome of NTM infection
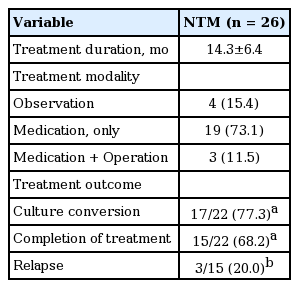
As shown in Table 4, the average treatment duration for NTM infection was 14.3 ± 6.4 months. Nineteen patients (73.1%) were treated with macrolide-based medications only, while additional lobectomy with medication was conducted in three patients (11.5%). Four patients (15.4%) were observed without treatment due to old age (n = 2) or refusal of treatment (n = 2). Among the 22 patients treated, 17 patients (77.3%) achieved a negative conversion of sputum culture, either with completion of treatment (n = 15) or on anti-NTM medication at last follow-up visit (n = 2). Five patients (22.7%) did not obtain negative culture conversion with maintenance of treatment (n = 2), treatment withdrawal due to adverse effects (n = 2), and death from pneumonia after lobectomy (n = 1). Pneumonia was the cause of death in one of the four patients not treated after 2 months of NTM infection. Among 15 patients who completed treatment, three experienced a relapse.
DISCUSSION
Previous studies have shown that patients with RA have an increased risk of NTM infection [8,17]. However, there have been few studies on the incidence or clinical characteristics of NTM infections in RA patients in South Korea. This may be due to the low incidence of NTM infection in South Korea [18]. Physicians are more likely to focus on TB infection because of its relatively high incidence. In our current study, the incidence of TB infection in RA patients was 329.5 per 100,000 patient-years, which is approximately three times higher than that of the general population of South Korea (108 per 100,000 patient-years, according to a 2012 World Health Organization report) [19]. However, the incidence of NTM infection in RA patients was 164.8 per 100,000 patient-years, implying that NTM infections are not rare among RA patients in South Korea.
In contrast to a previous study conducted in the United States [8], we found in our current analysis that the incidence of NTM infection was lower than that of TB infection in RA patients, which is not surprising considering that our present study was conducted in an area of intermediate TB burden. Nonetheless, NTM infection rates are increasing both in South Korea [20,21] and worldwide [22-24]. Therefore, NTM infection represents an increasing problem for patients with RA. Our study showed that NTM infection in RA patients has tended to increase after mid-2000. There are several explanations for this result. First, advances in laboratory techniques, such as liquid media culture, might contribute to the detection of NTM. Indeed, after our hospital initiated the use of liquid media in 2008, the frequency of NTM isolation increased. Second, an increase in the number of screening tests for TB infection before biological DMARDs might have resulted in an increased rate of NTM infection. During our present study period, NTM isolation in RA patients was the highest (23 of 111 NTM isolations) in 2008, a year with a high number of screening tests for mycobacterial infection. Furthermore, in six out of our 26 patients with NTM infection, NTM was detected for the first time through the screening test. Third, as mentioned above, NTM infection itself has been increasing in South Korea [20,21], and heightened awareness of this pathogen may have resulted in more frequent detection.
In patients treated with a TNF inhibitor, the risk of TB infection is better understood than the risk of NTM infection. However, there have been several reports showing higher rates of NTM infection in patients using TNF inhibitors [9,13]. In our present study, the proportion of patients using biological DMARDs at the time of NTM diagnosis (23.1%) was higher than that of TB infections (9.6%). Furthermore, the estimated incidence per 100,000 patient-years of NTM infection in RA patients with and without biological DMARDs was 655.7 and 134.5, while the incidence of TB infection in RA patients with and without biological DMARDs was 546.5 and 316.2, respectively. These data suggest that NTM infection could be more susceptible to biological DMARDs than TB infection because screening and prophylactic treatment has been performed only for latent TB infection before biological DMARDs therapy.
NTM and TB infections showed different clinical behaviors in our Korean patients with RA. All NTM infections in our study were pulmonary, whereas over a quarter of our TB cases were extrapulmonary. These results are consistent with a previous study of RA patients showing that most cases of NTM and TB infection were pulmonary, with a larger percentage of pulmonary infection in the NTM cases [8,17]. This result is potentially explained by the fact that RA patients have a high risk of chronic lung conditions, such as bronchiectasis, bronchiolitis, and ILD [25], that are known risk factors for pulmonary NTM disease [26-28]. Given the data in the current study, RA patients with NTM infection had a high incidence of concomitant ILD and previous history of TB infection, by which structural lung damage could be caused.
As a matter of fact, the percentage of ever-smoker in our RA patients with NTM and TB infection is higher than that in general RA patients. According to the previous reports, cigarette smoking have been suggested as a risk factor for pulmonary NTM infection [18] and TB infection [29]. In addition, smoking is also associated with lung diseases such as chronic obstructive pulmonary disease or lung cancer which are other risk factors for pulmonary NTM infection [18]. Therefore, high percentage of ever-smoker in our patients might be associated with increasing risk of NTM and TB infection.
In the present study, the levels of inflammatory markers such as ESR and CRP were elevated at the time of diagnosis of NTM or TB infection. It might be resulted from either high disease activity of RA or infection itself. Whereas the levels of ESR and CRP at the diagnosis of infection were increased than those at 6 months ago, the doses of DMARDs or corticosteroid were not changed during the period in most patients. Therefore, it is supposed that elevated levels of ESR and CRP could be a proxy for hidden infection in a case of stable underlying disease.
In our study, the cumulative steroid dose at the time of diagnosis was much greater in the patients with NTM infection than those with TB infection. And NTM infections tended to occur later, after the diagnosis of RA. These may imply that NTM infection developed more susceptibly in immune compromised RA patients. This is similar to human immunodeficiency virus-infected patients, in whom NTM infections usually occur at a later stage (a lower CD4 T-cell count) than TB infections [30,31].
There is geographic variability in the prevalence of NTM species that are responsible for disease [32]. In the United States and Japan, the two most common pathogens of NTM pulmonary infection are MAC and Mycobacterium kansasii [33,34]. M. kansasii and Mycobacterium malmoense are the most common species in England and Scotland, respectively. In South Korea, MAC is the most common species, followed by M. abscessus and the Mycobacterium fortuitum complex [35,36]. Our present investigation has revealed that, among RA patients, MAC was also the most common pathogen of pulmonary NTM infection, similar to previous reports from South Korea [35,36].
Our current findings showed favorable outcomes for NTM infection in RA patients (77.3% obtained negative culture conversion and 68.2% completed their treatment), similar to the results of previous studies of nonRA patients [37-39]. Moreover, among our six NTM patients who were treated with biological DMARDs, five obtained negative culture conversion (four complete treatments, one anti-NTM treatment at last follow-up visit), revealing good treatment outcomes similar to previous studies of RA patients receiving biological agents [12,16]. According to the 2007 ATS/IDSA official statement, patients with active NTM disease should receive TNF inhibitor only if they are also receiving adequate therapy for NTM infection. However, it has not been determined if TNF inhibitor should be stopped at the time of NTM infection diagnosis in patients undergoing TNF inhibitor therapy. In our present study, one patient continued to use etanercept with anti-NTM medication due to her high disease activity. She obtained a complete treatment status, suggesting that maintenance of TNF inhibitor in patients undergoing concomitant treatment with an adequate NTM treatment might be possible.
Our present study had several limitations of note. It was a retrospective and relatively small registry investigation at a single referral center. Furthermore, selection bias cannot be excluded. For example, RA patients with pulmonary comorbidity or high disease activity might have been more prevalent at our center than at a primary clinic, leading to possible overestimation of NTM infection incidence.
Conclusively, an incidence of 164.8 per 100,000 patient-years suggests that in RA patients, NTM infection is not rare in South Korea, where TB infection is endemic. A history of TB infection and concomitant ILD were found more commonly in patients with NTM infection compared to cases with TB infection. Therefore, physicians should be cautious about NTM infection, especially in RA patients with a history of TB infection and concomitant ILD.
KEY MESSAGE
1. In South Korea, nontuberculous mycobacteria (NTM) infection is not rare in rheumatoid arthritis (RA) patients, and infection rate are growing.
2. The most common species of NTM infection with RA patients was the Mycobacterium avium complex and the treatment outcome was generally favorable.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by the Asan Institute for Life Science (15-655).