p22phox C242T gene polymorphism and overt diabetic nephropathy: a meta-analysis of 1,452 participants
Article information
Abstract
Background/Aims
The p22phox C242T gene polymorphism (rs4673) may be linked to an increased susceptibility for overt diabetic nephropathy (ODN), but the study results are still inconclusive.
Methods
To explore the relationship between p22phox C242T gene polymorphism and ODN, the current meta-analysis of 707 ODN patients and 745 controls from five individual studies was conducted. The pooled odds ratio (OR) and its corresponding 95% confidence interval (CI) were evaluated by either a random or fixed effect model.
Results
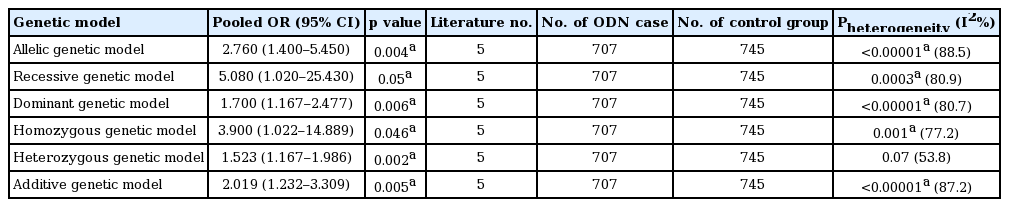
In our meta-analysis, a significant relationship between the p22phox C242T gene polymorphism and ODN was found under allelic (OR, 2.760; 95% CI, 1.400 to 5.450; p = 0.004), recessive (OR, 5.080; 95% CI, 1.020 to 25.430; p = 0.05), dominant (OR, 1.700; 95% CI, 1.167 to 2.477; p = 0.006), homozygous (OR, 3.900; 95% CI, 1.022 to 14.889; p = 0.046), heterozygous (OR, 1.523; 95% CI, 1.167 to 1.986; p = 0.002), and additive genetic models (OR, 2.019; 95% CI, 1.232 to 3.309; p = 0.005).
Conclusions
A positive correlation between p22phox C242T gene polymorphism and ODN risk was found. The T allele carriers of p22phox C242T gene polymorphism might be predisposed to ODN.
INTRODUCTION
Diabetic nephropathy (DN) is a severe complication of diabetes mellitus and is characterized by persistent proteinuria, decreased glomerular filtration rate, and hypertension with high morbidity and mortality due to cardiovascular diseases. Although DN can occur in both types of diabetes mellitus, studies indicate that the incidence of DN is higher in patients with type 1 diabetes mellitus (T1DM) than in those with type 2 diabetes mellitus (T2DM) (T1DM, 30% to 40%; T2DM, 20%). In the United States and other developed countries, overt diabetic nephropathy (ODN) has become the primary cause of end-stage renal failure, and 60% of patients undergoing hemodialysis in the United States suffer from ODN [1-3].
The underlying mechanism of ODN is multifactorial, resulting from the interactions among abnormal metabolism, hemodynamic changes, and various growth and genetic factors [4]. The principal pathological characteristic of glomerulosclerosis is the thickening of the glomerular basement membrane and the accumulation of the extracellular matrix [5]. Recently, Najafi et al. [6] have discovered that nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity is closely associated with p22phox (CYBA) C242T polymorphism (rs4673) because p22phox is an essential subunit for NADPH.
The p22phox gene, located in 16q24, spans 8.5 kb and contains six exons and five introns. In the C242T polymorphism (rs4673), the 242nd base cytosine (C) is substituted by thymine (T), resulting in the wild-type histidine (His) being replaced by tyrosine (Tyr) at the 72nd codon of the fourth exon. Given that the 72nd codon is the heme-binding site, the His > Tyr variant reduces the heme-binding affinity. Consequently, NADPH oxidase activity is affected, and the production of reactive oxygen species is significantly altered, ultimately contributing to renal cellular or organ injury [7].
Although many studies on the relationship between the p22phox C242T gene polymorphism and DN have been conducted, individual results are slightly contradictory. In 2003, Hodgkinson et al. [8] first reported that within the population of the United Kingdom, the frequency of the 242 TT genotype of the p22phox C242T gene polymorphism significantly increases in T1DM patients with ODN compared with those with retinopathy alone or uncomplicated diabetes (no microvascular disease after 20 years of diabetes duration), as well as normal healthy controls (33.3% vs. 6.5%, 5.7%, and 0.0%, respectively; p < 0.000001). Similarly, Liu et al. [9] found that the p22phox C242T gene polymorphism positively correlates with ODN and concluded that the T allele likely predisposes the Chinese population with T2DM to ODN. Yang et al. [10] drew the same conclusion in their study on the T2DM population in China. By contrast, Lim et al. [11] reported that the p22phox C242T gene polymorphism does not significantly increase the susceptibility to DN in another T2DM Chinese population.
The present meta-analysis involving 707 ODN cases and 745 controls was performed to explore the relationship between the p22phox C242T gene polymorphism and ODN (Supplementary Table 1) [12]. To avoid classifying subjects without significant renal impairment as cases and then erroneously enriching the cases with pathogenetic genotype, we only considered patients with ODN as cases in this meta-analysis.
METHODS
Publication search and inclusion criteria
Databases, including Web of Science, PubMed, Embase, China National Knowledge Infrastructure, and China Biological Medicine Database, were searched using the keywords ‘overt diabetic nephropathy,’ ‘p22phox,’ ‘C242T,’ and ‘polymorphism’ for our primary search of the literature. The retrieved studies were searched from 1980 (last research updated on July 1, 2015).
The inclusion criteria for our meta-analysis were as follows. (1) The studies must have evaluated the relationship between the p22phox C242T gene polymorphism and ODN. (2) ODN must be diagnosed by the presence of macroalbuminuria (albumin excretion rate ≥ 200 µg/min) or chronic renal failure treated by dialysis. Other renal diseases or other causes of proteinuria were excluded by renal biopsy. Furthermore, proteinuria must be measured more than once. In addition, the ODN patients must present a long duration of diabetes and be accompanied by diabetic retinopathy and other complications, or the ODN was confirmed by renal biopsy. (3) The studies must be published in official journals as case-control or cohort studies. (4) The Hardy-Weinberg equilibrium (HWE) must be followed by the studies.
Data extraction
Studies that failed to meet inclusion criteria or those with insufficient data were excluded. Data published multiple times was used in our analysis only once. The extracted data had to include the first author’s name, publication year, study region, number of genotypes, genotyping method, study design, matching criteria, and total number of ODN cases and controls.
Statistical analysis
Six genetic models, the allelic (T allele distribution frequency), recessive (TT vs. CC + CT), dominant (TT + CT vs. CC), homozygous (TT vs. CC), heterozygous (CT vs. CC), and additive genetic models (T vs. C), were adopted for our meta-analysis. The odds ratio (OR) corresponding to 95% confidence interval (CI) was used to compare the relationship between the p22phox C242T gene polymorphism and ODN. The heterogeneity between studies was calculated by chi-square-based q test with the threshold of significance set at p < 0.05 [13]. If heterogeneity were among the individual studies, the random-effects model (the DerSimonian and Laird method) would be used to estimate the pooled OR [14]. If not, a fixed-effects model (the Mantel-Haenszel method) would be adopted [15]. The Z test was used to determine the pooled OR with the threshold of significance was set at p ≤ 0.05 level.
The Fisher exact test was used to assess the HWE and significance was set at p < 0.05 level. The potential publication bias was estimated by funnel plot. The funnel plot asymmetry was assessed by Egger’s linear regression test on the natural logarithm scale of the OR with significance set as p < 0.05 [16]. The statistical analysis was performed using STATA version 11.0 software (Stata Corp., College Station, TX, USA).
RESULTS
Studies and populations
Our initial search of the literature retrieved 15 papers. Five of these papers met our inclusion criteria. Only one of these papers analyzed the relationship between the p22phox C242T gene polymorphism and ODN within the context of T1DM; the remaining four enrolled patients with T2DM. Among the 10 rejected papers, two papers were repeated publications, four papers were reviews, and four papers were unrelated to the subject at hand. In total, our data comprised 707 ODN patients and 745 controls (Table 1, Supplementary Fig. 1) [8-11,12,17]. Study regions comprised the United Kingdom, Brazil, and China. The populations were categorized into two ethnicities, namely, Caucasian and Chinese.
Pooled analyses
A significant relationship existed between the p22phox C242T gene polymorphism and ODN under allelic (OR, 2.760; 95% CI, 1.400 to 5.450; p = 0.004), recessive (OR, 5.080; 95% CI, 1.020 to 25.430; p = 0.05), dominant (OR, 1.700; 95% CI, 1.167 to 2.477; p = 0.006), homozygous (OR, 3.900; 95% CI, 1.022 to 14.889; p = 0.046), heterozygous (OR, 1.523; 95% CI, 1.167 to 1.986; p = 0.002), and additive genetic models (OR, 2.019; 95% CI, 1.232 to 3.309; p = 0.005) (Table 2, Figs. 1 and 2).
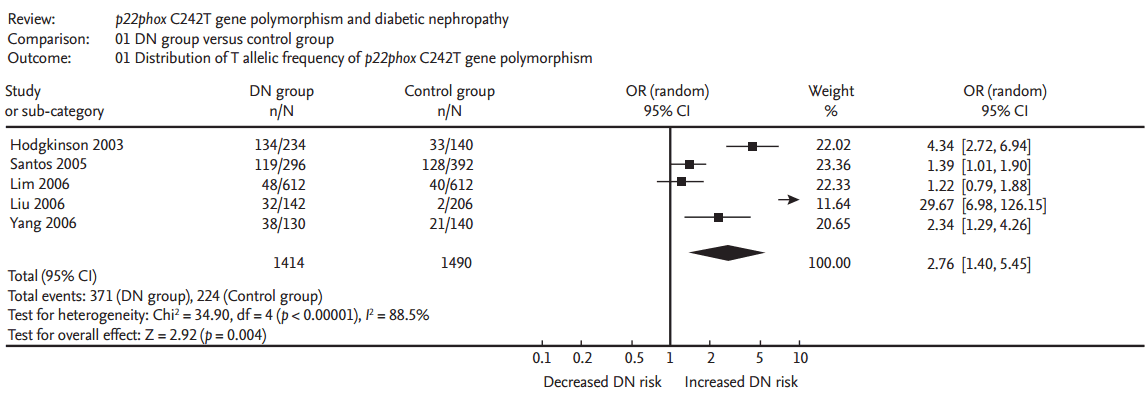
Forest plot of overt diabetic nephropathy associated with p22phox C242T gene polymorphism under allelic genetic model (distribution of T allelic frequency of p22phox gene). DN, diabetic nephropathy; OR, odds ratio; CI, confidence interval.
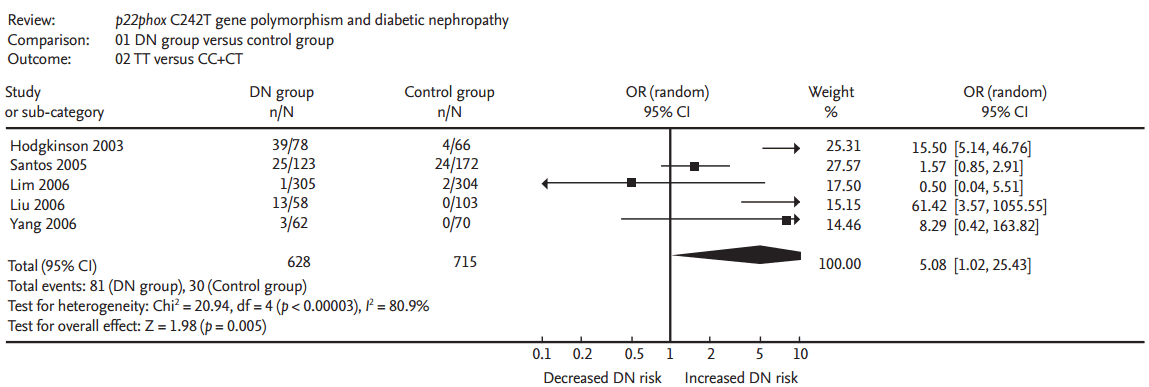
Forest plot of overt diabetic nephropathy (ODN) associated with p22phox C242T gene polymorphism under recessive genetic model (TT vs. CT + CC). DN, diabetic nephropathy; OR, odds ratio; CI, confidence interval.
After excluding the study on ODN from T1DM [8], the four remaining studies were adopted to perform the meta-analysis, and similar results were obtained. A significant relationship between the p22phox C242T gene polymorphism and ODN was detected under the allelic (OR, 2.320; 95% CI, 1.160 to 4.650; p = 0.02), dominant (OR, 2.317; 95% CI, 1.186 to 4.527; p = 0.014), homozygous (OR, 2.062; 95% CI, 1.128 to 3.767; p = 0.019), heterozygous (OR, 1.612; 95% CI, 1.210 to 2.165; p = 0.001), and additive genetic models (OR, 2.343; 95% CI, 1.152 to 4.764; p = 0.019). No significant relationship existed between the p22phox C242T gene polymorphism and ODN under the recessive genetic model (OR, 1.675; 95% CI, 0.952 to 2.947; p = 0.074) (Table 3, Figs. 3 and 4).

Summary of meta-analysis of association of p22phox C242T gene polymorphism and ODN confined to type 2 diabetes mellitus

Forest plot of p22phox C242T gene polymorphism associated with overt diabetic nephropathy (ODN) confined to type 2 diabetes mellitus under allelic genetic model (distribution of T allelic frequency of p22phox gene). IV, inverse variance method; CI, confidence interval; DN, diabetic nephropathy.
Bias diagnostics
The potential for publication bias of the individual studies was assessed using the funnel plot and Egger’s test. No visual publication bias was detected in the funnel plot (Fig. 5). Moreover, no significant difference was found in the Egger’s test, and this finding implied that no publication bias existed in the present meta-analysis, as shown by the allelic genetic model (T = −1.19, p = 0.321).
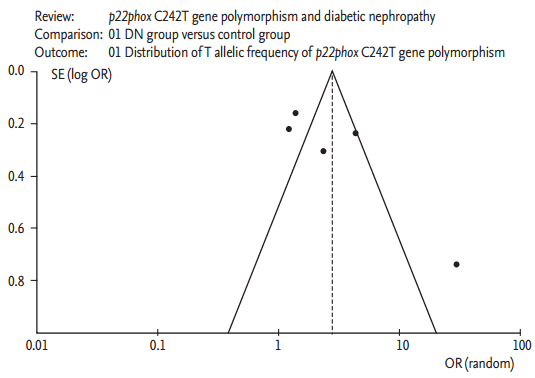
Funnel plot for studies of the association of overt diabetic nephropathy associated and p22phox C242T gene polymorphism under an allelic genetic model (distribution of T allelic frequency of p22phox gene). The horizontal and vertical axis correspond to the odds ratio (OR) and confidence limits. SE, standard error.
DISCUSSION
In this meta-analysis, a significant positive correlation was found between the p22phox C242T gene polymorphism and ODN under all six genetic models, namely, allelic (OR, 2.760), recessive (OR, 5.080), dominant (OR, 1.700), homozygous (OR, 3.900), heterozygous (OR, 1.523), and additive genetic models (OR, 2.019). Even after the study on ODN from T1DM was excluded, a similar conclusion was drawn. Thus, we believe that our results are significant. Additionally, the T allele carriers of the p22phox C242T gene polymorphism might predispose to ODN.
NADPH oxidase is a specific electron-transfer compound composed of five elements: p40phox, p47phox, p67phox, and cytochrome b588 (p22phox, gp91phox) [18]. Cytochrome b588 is the main element of NADPH and includes two subunits as heavy and light chains. The heavy chain is a 91 kDa glycoprotein, and the light chain is a 22 kDa polypeptide. The former is the core section of NADPH oxidase, and the latter is the key position of NADPH-producing oxygen radicals [19]. NADPH oxidase is a key enzyme that produces superoxides. In particular, p22phox plays a crucial role in NADPH normal function and O2− generation.
Whether the p22phox C242T gene variant contributes to vascular damage by increasing or decreasing NADPH oxidase activity was previously unclear. In 2000, Guzik et al. [20] found that the p22phox 242T allele is associated with reduced NADPH oxidase activity in human blood vessels and concluded that genetic variation in NADPH oxidase components might play a significant role in modulating superoxide production in human atherosclerosis. By contrast, Perianayagam et al. [7] found that patients with acute renal failure, who also exhibited the p22phox 242TT polymorphism, present higher levels of plasma nitrotyrosine than those with the wild-type genotype. This finding indicates an increase in NADPH activity induced by the T allele [7]. Enzyme activity is modulated by many factors in vivo, and the different experimental approaches of these two studies may contribute to the varying results [21]. NADPH activity is likely increased by the genetic polymorphism, considering that the latter experiment was performed in vivo.
The differential effects of the C242T variants on other biological processes, such as the upregulation of antioxidant defenses, inflammation, lipid peroxidation, gene expression, and apoptosis, can serve as potential explanations for their involvement in vascular damage [22,23]. In 2003, Hodgkinson et al. [8] found that NADPH oxidase with the polyol pathway may contribute to the pathogenesis of DN.
In the current meta-analysis, we selected the cases of ODN to avoid enrolling incipient renal impairment, but this selection can also enrich the group of cases. A significant heterogeneity among the individual studies might be associated with the gene variant distribution difference in diverse ethnicities. Additionally, DN may behave differently in T1DM and T2DM, but the present meta-analysis reached the same conclusion even after the study regarding ODN and T1DM was excluded. In 2003, Ji et al. [24] found that the p22phox C242T allele and genotype distribution in the Chinese population are similar to those in the Japanese population and much lower than those in Caucasians and Indians [23-26]. Given that only five manuscripts were included, meta-regression was not performed to explore the heterogeneity source in the current meta-analysis.
Despite the robust results of this analysis, which is the first to explore the association between the p22phox C242T gene polymorphism and ODN, some limitations still affect this meta-analysis. First, few individual studies were included in this meta-analysis, and large-scale studies on the relationship of ODN to the p22phox C242T gene polymorphism are lacking. Second, NADPH oxidase activity can also be influenced by other genetic polymorphisms, such as A640G (rs1049255), A8897T (rs4782390), and G383A (rs3794624).
In conclusion, we found a positive correlation between the p22phox C242T gene polymorphism and ODN susceptibility. The T allele carriers may be ascribed to the increased susceptibility. This conclusion may facilitate individual diagnosis of ODN and guide therapeutic strategies. Nevertheless, considering the above limitations, further studies are necessary to elucidate the relationship between the p22phox C242T gene polymorphism and ODN susceptibility.
KEY MESSAGE
1. The essential subunit for nicotinamide adenine dinucleotide phosphate, p22phox C242T gene polymorphism (rs4673) was positively correlated with overt diabetic nephropathy (ODN).
2. The T allele carriers of p22phox C242T gene polymorphism might be predisposed to ODN.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (NSFC 81100073 to Dr Yan-yan Li), Excellent Young and Middle-Aged Teachers Assistance Program of Nanjing Medical University for Dr Yan-yan Li (2013-2015, JX2161015034), Jiangsu Overseas Research & Training Program for University Prominent Young & Middle-aged Teachers and Presidents (2014), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). Thank all our colleagues working in the Department of Geriatrics, the First Affiliated Hospital of Nanjing Medical University.