The role of 18F-fluorodeoxyglucose positron emission tomography in the assessment of disease activity of adult-onset Still’s disease
Article information
Abstract
Background/Aims
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has been suggested as a reliable imaging technique for monitoring of disease activity in patients with adult-onset Still’s disease (AOSD). Therefore, we investigated the clinical significance of 18F-FDG PET/CT in Korean AOSD patients.
Methods
Thirteen AOSD patients were included in the study. The PET/CT images were evaluated with visual and semiquantitative method using standardized uptake values (SUVs).
Results
The presence of increased 18F-FDG uptake was noted in 90% of clinically active AOSD patients. 18F-FDG uptake was located in the lymph node, spleen, and bone marrow. Visual grade and SUV intensity of lymph node was significantly correlated with the systemic score of AOSD. Visual grade of spleen was significantly correlated with the systemic score, erythrocyte sedimentation rate (ESR), and ferritin. Additionally, visual grade and SUV intensity of bone marrow was significantly correlated with the systemic score, ESR, leukocyte, and neutrophil.
Conclusions
Visual grade and SUV intensity of lymph node, spleen, and bone marrow on 18F-FDG PET/CT scan showed significant correlations with known disease activity markers. The data suggest that 18F-FDG PET/CT scan may be a useful imaging technique for evaluation of disease activity in AOSD patients.
INTRODUCTION
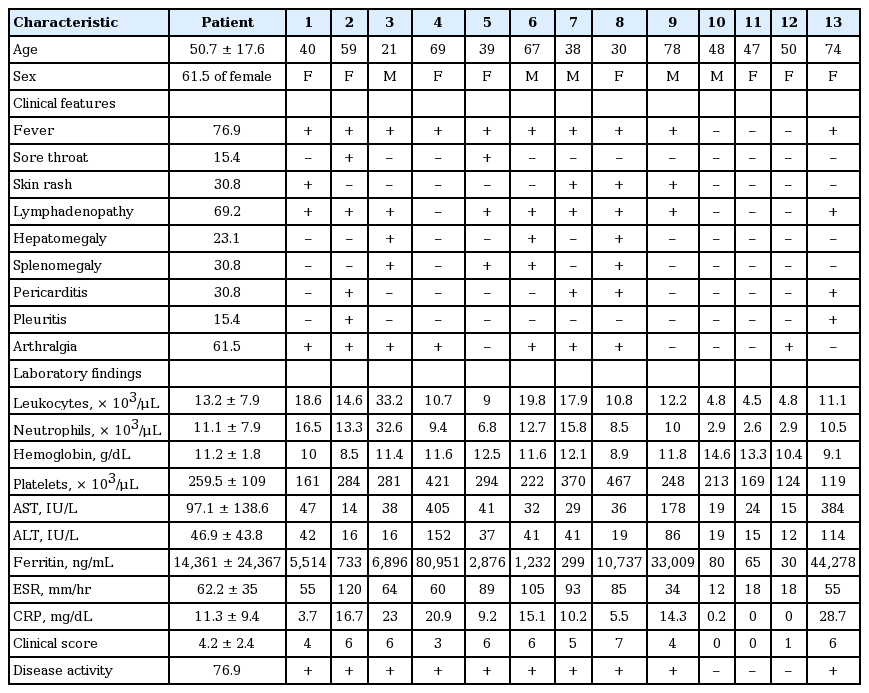
Adult-onset Still’s disease (AOSD) is a systemic inflammatory disease of unknown etiology characterized by spiking fever, arthritis, evaneascent skin rash, hepatosplenomegaly, and lymphadenopathy [1]. AOSD is one cause of fever of unknown origin (FUO) and about 5% patients with FUO have AOSD [2]. Howerver, the symptoms and laboratory results are not disease specific, and the clinical features overlap with autoimmune disorder, infections, or malignancies. Therefore, the spectrum of differential diagnoses is wide and includes infectious, neoplastic, and autoimmune disorder, and these diseases should be ruled out before the diagnosis of AOSD [3,4]. Accurate determination of disease activity is also difficult. The commonly used biomarkers for AOSD are nonspecific inflammatory markers including erythrocyte sedimentation rate (ESR), serum C-reactive protein (CRP), and ferritin. Furthermore, imaging studies such as abdominal computed tomography (CT) and echocardiogram can be used only for the dectection of nonspecific clincial features including lymphadenopathy, splenomegaly, and pericarditis.
18F-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) is an useful imaging tool that can visualize the metabolic status of the whole body. It is well established as a diagnostic modality in malignant diseases, such as lung cancer and lymphoma. Additionally, it can be used for evaluating infectious and inflammatory diseases by targeting the increased glucose uptake of inflammatory cells. Several studies showed the clinical value of using 18F-FDG PET/CT scans to aid in the evaluation of patients with rheumatic diseases including sarcoidosis, large-vessel arteritis, and Sjögren’s syndrome [5-8].
18F-FDG PET/CT may have a potential role in the diagnosis and monitoring of AOSD [9-15]. However, several studies were case reports, and there has been only one study shown correlation between disease activity and 18F-FDG PET/CT results. Therefore, we investigated the clinical significance of 18F-FDG PET/CT with systemic scores in Korean AOSD patients.
METHODS
Subjects
Thirteen AOSD patients were included in the study. All AOSD patients referred to Ajou University Hospital for a 18F-FDG PET/CT between 2008 and 2013 were retrospectively analyzed. 18F-FDG PET/CT was performed on 10 patients to evaluate lymphadenopathy or to rule out of malignancy when the initial systemic inflammatory symptoms suggested AOSD. Three patients received 18F-FDT PET/CT for cancer screening during treatment of AOSD, and their disease activities were inactive. AOSD patients were diagnosed according to Yamaguchi’s criteria after exclusion of common infections and hematological and autoimmune diseases [16].
Information on medical histories, clinical symptoms, and physical examinations was entered into a database when 18F-FDG PET/CT was done. Each patient also underwent a series of laboratory tests, including complete blood count, ESR, CRP, rheumatoid factor, anti-nuclear antibody, ferritin (normal 13 to 150 ng/mL for women and 30 to 400 ng/mL for men), and liver function tests. AOSD disease activity was evaluated according to the method described by Pouchot et al. [3], which assigns a score from 0 to 12 and adds 1 point for each of the following manifestations: fever, typical rash, pleuritis, pneumonia, pericarditis, hepatomegaly or abnormal liver function tests, splenomegaly, lymphadenopathy, leukocytosis ≥ 15,000/mm2, sore throat, myalgia, and abdominal pain. We defined resolution of disease activity of AOSD with this score ≤ 2. This study was approved by the Ajou University Hospital Institutional Review Board (AJIRB-MED-OBS-13-234).
18F-FDG PET/CT acquisition
After fasting for least 6 hours, patients were administered 370 MBq of 18F-FDG intravenously. All patients were instructed to rest comfortably for 60 minutes and to urinate before scanning. Whole-body PET/CT images were obtained with a discovery ST scanner (GE Healthcare, Milwaukee, WI, USA). Seven to eight frames (3 min/frame) of emission PET data were acquired in three-dimensional mode after a non-contrast CT scan from the base of the skull to the upper thigh (tube rotation time of 1 second per revolution, 120 kV, 60 mA, 7.5 mm per rotation, and an acquisition time of 60.9 second for a scan length of 867 mm). Emission PET images were reconstructed using an iterative method (ordered-subsets expectation maximization with two iterations and 30 subsets, field of view = 600 mm, slice thickness = 3.27 mm) and attenuation-corrected with non-contrast CT.
18F-FDG PET/CT image interpretation and analysis
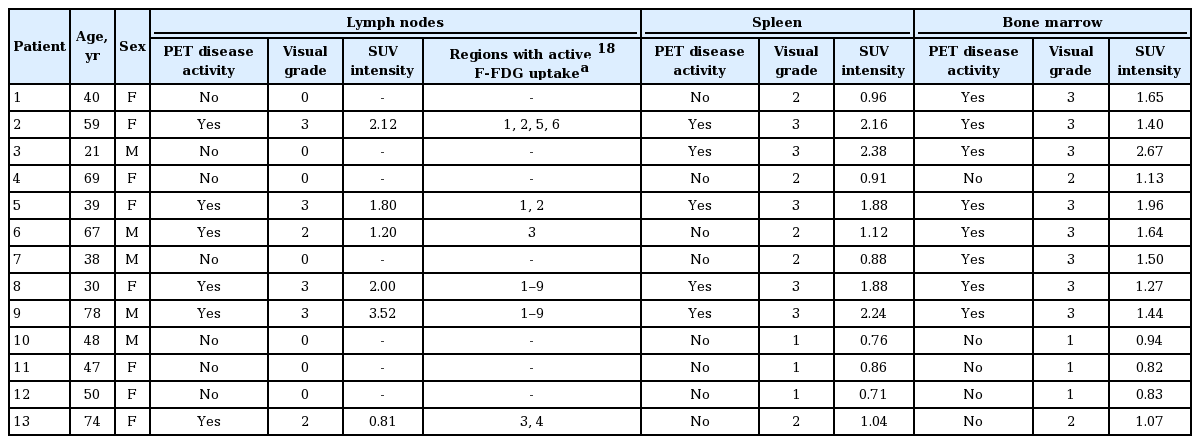
Transaxial, saggital, coronal, and maximum intensity projection images of 18F-FDG PET/CT were assessed visually on an AW workstation version 4.4 (General Electric Healthcare, Chicago, IL, USA) by an experienced nuclear medicine physician blinded to the results of clinical data. 18F-FDG uptake was graded on a scale from 0 to 3: 0, no uptake; 1, below liver uptake; 2, corresponding to liver uptake; and 3, above liver uptake. Grade ≥ 2 considered active 18F-FDG uptake for lymph nodes and grade ≥ 3 deemed active PET diseases for spleen and bone marrow [17]. For the semiquantitative analysis of 18F-FDG uptake, standardized uptake values (SUVs) were calculated based on injected dose and body weight. Three dimensional spherical volume of interest (7.11 cm3) were placed on the lymph nodes, spleen, and bone marrows and maximum SUVs (SUVmax) were recorded. If there were multiple lymph nodes uptakes in one patient, we selected one lymph node with the most intense 18F-FDG uptake as a representative value. When the lesion could not be visualized due to a lack of 18F-FDG uptake for lymph nodes, an SUV of 0 was recorded. SUV intensity was calculated by dividing the SUVmax of lesions (lymph nodes, spleen, and bone marrows) by that of liver (segment VIII) to minimize the effect of time from injection to acquisition and to minimize the effect of blood glucose levels proposed by Lee et al. [8]. The same process was repeated a minimum of 6 months later, and these two readings were compared to estimate intrareader reliability.
Statistical analysis
The statistical analysis was performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). A p < 0.05 was regarded as statistically significant. The data are shown as mean ± standard deviation or median and interquartile range, where appropriate. The correlations between 18F-FDG PET/CT results and disease activity markers of AOSD were evaluated with a Spearman’s correlation test. Intrareader reliability was measured using the intraclass correlation coefficient (ICC), as generated by two-way random effect model and an absolute agreement definition.
RESULTS
Clinical characteristics of the patients
The mean age of the AOSD patients was 50.8 ± 17.6 years, and females comprised 61.5%. The main clinical symptoms in AOSD patients included high spiking fever (76.9%), skin rash (30.8%), sore throat (4.7%), arthralgia (53.8%), and lymphadenopathy (69.2%). Ten patients (76.9%) had clinically active disease. Table 1 lists the clinical features, laboratory findings, and clinical disease activities of each patient.
18F-FDG PET/CT results in 13 AOSD patients
Table 2 shows the 18F-FDG PET/CT results of each patient. Intrareader agreement for PET was perfect with ICCs of 1.000. The presence of increased 18F-FDG uptake was noted in 90% of clinically active AOSD patients (n = 10). 18F-FDG uptake was located to lymph node, spleen, and bone marrow. Also, there was no uptake of 18F-FDG in clinically inactive AOSD patients (n = 3). Among the 18F-FDG PET/CT scan of 10 clinically active AOSD patients, six scan (60%) showed active 18F-FDG uptake in at least one lymph node region (median SUV intensity, 1.9 [1.0 to 2.47]). Lymph nodes were located in right cervical (n = 4/6), left cervical (n = 4), right axilla (n = 4), left axilla (n = 3), right pulmonary/mediastinal (n = 3), left pulmonary/mediastinal (n = 3), abdominal/mesenteric (n = 2), right inguinal/femoral (n = 2), or left inguinal/femoral (n = 2) sites. Five scans (50%) showed active 18F-FDG uptake of spleen with a median SUV intensity of 2.16 (1.88 to 2.31). Furthermore, 18F-FDG bone marrow uptake was observed in 80% of patients (8/10) with clinically active AOSD patients with a median SUV intensity of 1.57 (1.41 to 1.88).
Correlation of 18F-FDG PET/CT with clinical disease activity of AOSD
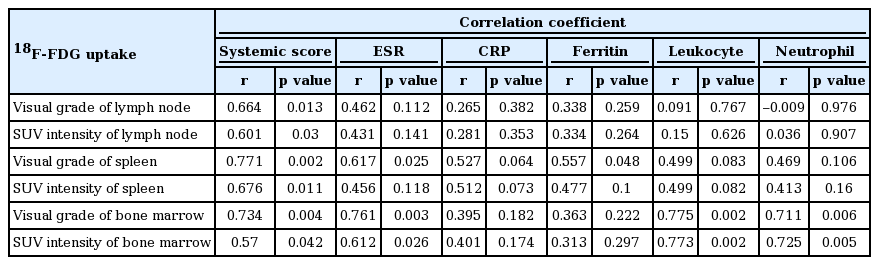
Visual grade of lymph node was significantly correlated with the systemic score of AOSD (r = 0.664, p = 0.013). SUV intensity of lymph node was correlated with the systemic score (r = 0.601, p = 0.03). Visual grade of spleen was significantly correlated with the systemic score (r = 0.771, p = 0.002), ESR (r = 0.617, p = 0.025), and ferritin (r = 0.557, p = 0.048). SUV intensity of spleen was correlated with the systemic score (r = 0.676, p = 0.011). Also, visual grade of bone marrow was significantly correlated with the systemic score (r = 0.734, p = 0.004), ESR (r = 0.761, p = 0.003), leukocyte (r = 0.775, p = 0.002), and neutrophil (r = 0.711, p = 0.006). SUV intensity of bone marrow was correlated with the systemic score (r = 0.57, p = 0.042), ESR (r = 0.612, p = 0.026), leukocyte (r = 0.773, p = 0.002), and neutrophil (r = 0.725, p = 0.005) (Table 3).
DISCUSSION
To examine the clinical significance of 18F-FDG PET/CT, we reviewed 18F-FDG PET/CT results in AOSD patients and evaluated the results with disease activity markers. Ninety percent of active AOSD patients had 18F-FDG uptake occurring in lymph node, spleen, or bone marrow and that this uptake was significantly associated with clinical disease activity of AOSD. Additionally, 18F-FDG uptak correlated significantly with inflammatory markers such as ESR, CRP, or ferritin.
18F-FDG uptake is also elevated in infectious and non-infectious inflammatory lesions, not only tumor cells. Reports have suggested that 18F-FDG PET may aid in the diagnosis or monitoring disease activity of inflammatory diseases such as large vessel arteritis, sarcoidosis, and Crohn disease [5,6,8,18-20]. In addition, 18F-FDG PET could be helpful in making a final diagnosis in patients with FUO [21-23]. Some studies showed the clinical role of 18F-FDG PET for identifying the causes of FUO in patients with AOSD [22,23].
Case reports have assessed 18F-FDG PET in AOSD [9-11,13,24,25]. The AOSD patients in previous reports were evaluated with 18F-FDG PET to rule out other febrile diseases such as infection, vasculitis, or malignancies or to work up lymphadenopathy. Most patients displayed increased uptake of 18F-FDG at multiple lymph nodes, spleen, or bone marrow [9,11,13,15,25]. One case showed an elevated 18F-FDG level in the sacroiliac joints because of arthritis associated with AOSD [24]. In another case, increased 18F-FDG uptake was shown in the carotids, the wrist, and the large vessels of the legs [12]. A recent report showed diffuse 18F-FDG uptake on quadriceps muscle bilaterally and a small uptake on the iliac bone [11]. In the present study, nine patients among 10 active AOSD patients showed increased 18F-FDG uptake at lymph node, spleen, or bone marrow. Four patients had increased 18F-FDG uptake at all sites, one at only lymph node, two at only bone marrow, one at lymph node and bone marrow, and one at spleen and bone marrow. 18F-FDG uptake of other site except lymph node, spleen, and bone marrow was not shown in our patients. In addition, there was no uptake of 18F-FDG in clinically inactive AOSD patients.
Increased splenic uptake is observed in human immunodeficiency virus infection and lymphoma. Diffusely increased splenic uptake may also be present in sarcoidosis, malaria, and many inflammatory or hematopoietic diseases [26]. Furthermore, one study showed the relationship between hematologic parameters and bone marrow and splenic uptake of 18F-FDG in PET imaging [27]. In the present study, diffuse 18F-FDG uptake in bone marrow and spleen was shown in the AOSD patients, consistent with previous studies [9-11,13,15,25]. In addition, the frequency of 18F-FDG uptake of bone marrow is higher than that of spleen or lymph node. Therefore, this study showed that 18F-FDG diffuse uptake in bone marrow and spleen could be common feature of AOSD in 18F-FDG PET.
Only one study showed a correlation between clinical activity and 18F-FDG PET results in AOSD [14]. They reported that no significant correlation was found between SUVmax in each lesion and the laboratory data, except for a significant correlation between lactate dehydrogenase and spleen SUV. And, three studies showed initial and follow-up 18F-FDG PET results of their cases [9-11]. All studies showed significant improvement (decreased SUV levels) of the initially abnormally sized radioactive lesions in follow-up images after treatment. In the present study, although there were no follow-up 18F-FDG PET/CT results in our AOSD patients, we enrolled 10 active AOSD patients and three inactive AOSD patients. We evaluated a correlation between the results of 18F-FDG PET/CT and clinical activity. Visual grade and SUV intensity of lymph node was significantly correlated with the systemic score of AOSD. Visual grade of spleen was significantly correlated with the systemic score, ESR, and ferritin. Additionally, visual grade and SUV intensity of bone marrow was significantly correlated with the systemic score, ESR, and leukocyte. Therefore, these results suggest that 18F-FDG PET/CT can provide reliable clinical information for monitoring the disease activity. In the present study, we assessed 18F-FDG uptake using both visual and semiquantitative (standard uptake value intensity) methods proposed previously [8], and checked the number of lymph node lesions with active 18F-FDG uptake. We found similar significant associations between clinical disease activity and the two methods (visual and semiquantitative methods).
This study had several limitations. It was retrospective and so may have weaknesses that include selection bias. Additionally, it was a cross-sectional study of small sample size conducted without follow-up scan. Further large-scale prospective studies are needed to determine the usefulness of 18F-FDG PET/CT scan for the diagnosis and evaluation of disease activity of AOSD. However, our study showed a significant correlation between clinical disease activity and 18F-FDG uptake in AOSD. In addition, we evaluated the association between 18F-FDG uptake and several inflammatory markers.
In conclusion, the presence of increased 18F-FDG uptake in lymph node, spleen, or bone marrow was noted in 90% of clinically active AOSD patients. Additionally, visual grade and SUV intensity of lymph node, spleen and bone marrow on 18F-FDG PET/CT scan showed significant correlations with known disease activity markers. These data suggest that 18F-FDG PET/CT scan may be a useful imaging technique for evaluation of disease activity in AOSD patients. For clinical usefulness of 18F-FDG PET, further prospective study with large sample size is needed.
KEY MESSAGE
1. The presence of increased 18F-f luorodeoxyglucose (18F-FDG) uptake in lymph node, spleen, or bone marrow was noted in 90% of clinically active adult-onset Still’s disease (AOSD) patients.
2. 18F-FDG positron emission tomography/computed tomography scan may be a useful imaging technique for evaluation of disease activity in AOSD patients.
Notes
No potential conflict of interest relevant to this article was reported.