A prospective cohort study of latent tuberculosis in adult close contacts of active pulmonary tuberculosis patients in Korea
Article information
Abstract
Background/Aims:
The objective of this prospective study was to evaluate the diagnosis and treatment of latent tuberculosis infection (LTBI) in adult close contacts of active pulmonary tuberculosis (TB) patients in Korea.
Methods:
Adult close contacts of active pulmonary TB patients were recruited at a regional tertiary hospital in Korea. The participants were tested for LTBI using the tuberculin skin test (TST) and/or QuantiFERON-TB Gold (QFT-G) test. LTBI patients, who consented to treatment, were randomly assigned to receive isoniazid for 9 months (9INH) or rifampin for 4 months (4RIF).
Results:
We examined 189 adult close contacts (> 18 years) of 107 active pulmonary TB patients. The TST and QFT-G were positive (≥ 10 mm) in 75/183 (39.7%) and 45/118 (38.1%) tested participants, respectively. Among 88 TST or QFT-G positive LTBI participants, 45 participants were randomly assigned to receive 4RIF (n = 21) or 9INH (n = 24), respectively. The average treatment duration for the 4RIF and 9INH groups was 3.3 ± 1.3 and 6.1 ± 2.7 months, respectively. Treatment was completed in 25 participants (4RIF, n = 16; 9INH, n = 9). LTBI participants who accepted treatment were more likely to be women and have more cavitary lesions on the chest radiographs of index cases and positive TST and QFT-G results compared to those who refused treatment.
Conclusions:
About 40% of adult close contacts of active pulmonary TB patients had LTBI; about 50% of these LTBI participants agreed to treatment.
INTRODUCTION
Korea has an intermediate tuberculosis (TB) burden, and the annual incidence of new active TB cases in this country is estimated to be 71.3 in 100,000 people [1,2]. Recently, there have been reports regarding the latent tuberculosis infection (LTBI) rate among Korean healthcare workers and individuals who have been in close contact with active pulmonary TB patients [3-6].
People exposed to TB are at risk of acquiring either LTBI or active TB. Although those with LTBI do not show signs, symptoms, or radiographic evidence of TB disease [7], they have a 10% lifetime risk of developing active TB. Half of all active TB cases occur within the first 2 years following TB exposure [8]. Therefore, early diagnosis and treatment of LTBI could be a useful strategy for reducing the long-term burden of TB, especially among people who have been in close contact with active TB patients.
According to the Korean guidelines for TB reported in 2014, the LTBI test is recommended for close contacts of active pulmonary TB patients and treatment should be considered for those < 35 years of age [9]. The diagnosis and treatment of LTBI in Korean adult close contacts of active pulmonary TB patients have not yet been reported. Here, we have reported our experience regarding the diagnosis and treatment of adults who contracted LTBI from close contact with active TB patients.
METHODS
Subjects and study design
The data were collected during an international multicenter trial of LTBI entitled, “A randomized clinical trial comparing 4RIF vs. 9INH for treatment of latent tuberculosis infection: effectiveness” (registration number; NCT00931736). The study protocol and inclusion and exclusion criteria of participants are described in the website (https://clinicaltrials.gov/ct2/show/study/NCT00931736). According to study protocol, the inclusion criterion for treatment was a tuberculin skin test (TST) reaction of ≥ 5 mm for close contacts of active pulmonary TB patients.
This current study was conducted at a single regional tertiary hospital in Korea, which was part of the international multicenter trial. This study was approved by the Institutional Review Board of Gyeongsang National University Hospital, Korea (IRB No. GNUHIRB-2009-042). Informed consent was obtained from the enrolled participants.
From October 2009 to August 2013, we recruited individuals (aged > 18 years), who were considered to be close contacts of active pulmonary TB patients. These individuals were asked to be volunteers for the LTBI test at the Gyeongsang National University Hospital, Korea. A “close contact” was defined as a person who had contact, for at least 4 hours/week, with a patient(s) with active pulmonary TB during an infectious period, and was confirmed by an acid-fast bacilli (AFB) smear and/or AFB culture test. We excluded health care workers, those diagnosed with active TB after chest radiography, and those who were in contact with patients with drug-resistant TB as indicated by the drug susceptibility profile. Participants underwent a TST and/or QuantiFERON-TB Gold (QFT-G, Cellestis Ltd., Carnegie, Australia) test after signing informed consent. If both tests were conducted during the same day, the TST was performed first. We assessed risk factors for contracting LTBI, including age, sex, history of bacilli-Calmette-Guerin (BCG) vaccination, history of pulmonary TB, cohabiting with a TB patient in the same house or room, presence of cough, AFB smear status of the index case, and cavitary lesions on the chest radiographs of the index case.
TST and QFT-G
The TST was performed according to the Mantoux method (Statens Serum Institute, Copenhagen, Denmark). The participants were injected intradermally with 0.1 mL of tuberculin purified protein derivative RT 23 SSI (2TU) by a trained research nurse, and the transverse diameter (mm) of the induration at the injection site (inner forearm) was measured 48 to 72 hours after injection [10,11]. The QFT-G tests were performed by a specialized laboratory company (Samkwang Medical Laboratories, Seoul, Korea) according to the manufacturer’s instructions. We incubated whole blood at 37ºC for 16 to 24 hours with TB-specific antigens containing an early-secreted antigenic target 6 protein and culture filtrate protein 10, and then measured the concentration of the released interferon gamma in the supernatant solution using an enzyme-linked-immunosorbent serologic assay [12,13].
Treatment of LTBI
In this study, LTBI was defined as a positive result for either TST (≥ 10 mm) or QFT-G, and participants who tested positive for LTBI were randomly allocated to receive isoniazid for 9 months (9INH; 9 months of 5 mg/kg isoniazid up to 300 mg/day) or rifampin for 4 months (4RIF; 4 months of 10 mg/kg rifampin up to 600 mg/day). Participants who showed resistance to isoniazid or rifampin during the drug sensitivity test were excluded from the study. Participants were allowed to withdraw from treatment if they choose to do so. We also compared LTBI patients who accepted and refused treatment.
Statistical analysis
Descriptive data were presented as numbers and percentages; continuous data were indicated by mean ± standard deviation or median (range). Categorical variables were compared using the chi-square test or Fisher exact test, as appropriate. Continuous variables were compared using the parametric t test or the Mann-Whitney U test. Differences were considered statistically significant if p < 0.05. Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Characteristics of study participants
We identified 348 adults who had close contact with 128 active pulmonary TB patients who were willing to test for LTBI with the TST and/or QFT-G. We excluded health care workers (n = 61) and those aged < 18 years (n = 7). Individuals were also excluded if the TB patient with whom they had close contact with had the following: (1) no Mycobacterium tuberculosis (MTB) growth (n = 31) on their sputum or bronchial washing fluid samples; (2) positive test for mono- or multi-drug resistance (n = 21); and (3) no documented results of a drug sensitivity test for MTB (n = 40) (Fig. 1). After these exclusions, we enrolled 189 participants who were close contacts of 107 active TB patients. The mean number of tested participants per active TB patient was 1.8 ± 1.2 (range, 1 to 7). The number of participants who underwent the TST, QFT-G, and both TST and QFT-G tests were 183, 118, and 112 participants, respectively. Seventy-six participants were tested with TST and QFT-G on the same day, and the mean time interval for undergoing both tests was 3.1 ± 2.2 days (range, 1 to 14). Two participants were tested with the QFT-G 7 and 14 days after the TST.

Flow chart of the study. Diagnosis of latent tuberculosis (TB) assessed by the tuberculin skin test (TST) and/or QuantiFERON-TB Gold (QFT-G) test. MTB, Mycobacterium tuberculosis; INH, isoniazid; RIF, rifampin.
The mean age of participants was 48.7 ± 15.7 years; 130 (68.8%) were women and 140 were household contacts (74.1%). Most of the participants (n = 170, 89.9%) had a scar from BCG vaccination (90.7% and 88.1% in the TST and QFT-G groups, respectively). A history of pulmonary TB was noted in 8.2% and 7.6% of the participants in the TST and QFT-G groups, respectively (Table 1). Comorbid diseases, including hypertension, diabetes, cardiovascular, chronic liver, and thyroid disease, were reported in 28.9% and 23.7% of participants in the TST and QFT-G groups, respectively. More than half of the participants in both the TST and QFT-G groups were household close contacts (73.8% and 72.9% in the TST and QFT-G groups, respectively) and resided in the same room (41.5% and 43.2% in the TST and QFT-G groups, respectively). In index cases, the rate of positive AFB smears obtained from the sputum or bronchial washing fluid in active pulmonary TB patients was 79.8% and 77.9% in the TST and QFT-G groups, respectively. Coughing as a respiratory symptom was observed in about 60% of active pulmonary TB patients. About 40% of active pulmonary TB patients were observed to have cavitary lesions in their chest radiographs.
TST and QFT-G
Seventy-five of the 183 participants who underwent TST (≥ 10 mm, 40.9%) and 45 of the 118 participants who underwent QFT-G testing (41.0%) had positive results. There were no other significant differences in the clinical characteristics of TST-positive and TST-negative participants, including the mean age, proportion of participants with visible BCG vaccination scars, history of TB, existence of comorbid illnesses, proportion of participants who had never smoked, household contacts (or residing in the same room), AFB smear status, presence of cough, or chest radiograph-confirmed cavitary lesion(s) in the index cases with LTBI. Similar findings were also observed in QFT-G-positive and QFT-G-negative participants (Table 2).
Treatment of LTBI
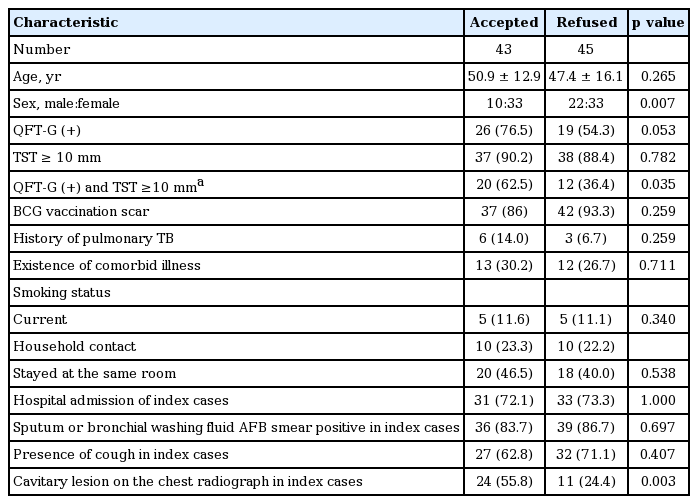
Among the 88 participants with TST or QFT-G positive results, 43 agreed to treatment. Forty-two of the 45 participants did not consent to treatment, two had signs of active TB on their chest radiograph, and one did not visit the hospital after TST/QFT-G testing. There were no differences in age, smoking status, history of TB, presence of cough in index cases, and household contacts (or residing in the same room) between those who agreed to and refused treatment. However, a higher percentage of women (76.7% vs. 60.0%, respectively) and those with cavitary lesions on chest radiograph in index cases (55.8% vs. 24.4%, respectively) accepted rather than refused treatment. Among the 63 participants who underwent testing for both TST and QFT-G, a positive result for both tests was more common in those who accepted treatment compared to those who refused treatment (62.5% vs. 36.4%, respectively) (Table 3). Twenty of the 43 participants who agreed to treatment had positive results for both TST and QFT-G, while eight and six participants tested positive for only TST and QFT-G, respectively. Those who consented to treatment were randomly assigned to receive 9INH (n = 22) or 4RIF (n = 21). The median duration of treatment for participants who underwent 9INH and 4RIF was 6 months (range, 1 to 9) and 4 months (range, 1 to 4), respectively. Nine of the 22 patients in the 9INH group and 16 of the 21 participants in the 4RIF group completed treatment (Fig. 2).
DISCUSSION
To the best of our knowledge, this was the first study in which the diagnoses and treatment experiences have been reported for adults who contracted LTBI after having close contact with active pulmonary TB patients in Korea. About 40% of adult close contacts of active pulmonary TB patients were diagnosed as having LTBI, and about 50% of these LTBI participants agreed to treatment. Based on the Korean guideline of ≥ 10 mm for TST positivity, 38.4% of our participants tested positive for LTBI. The incidence of a positive TST result for close contacts of active TB patients in Korea is variable. Previously reported incidence rates for a positive TST result include 47.1% (n = 90/206) by Park [6], 71% by Kang et al. [14], and 14.7% (n = 270/1826) during a school TB outbreak by Song et al. [5] In other countries, the incidence rate of LTBI among close contacts of active TB patients, as assessed by TST, also varied considerably from 27.9% to 93.0% [15].
The interferon gamma release assay (IGRA) has been known as an accepted specific test to detect LTBI, and therefore, it is widely used in Korea. In previous studies that involved the assessment of the incidence of LTBI in Korea using IGRA, a positive QFT-G result was observed in 11.1% (n = 203/1826) of participants who had close contact with active TB patients [5]. The percentages of positive IGRA results observed in previous studies (44% and 53.5% of participants in the studies by Kang et al. [14] and Jo et al. [16], respectively) were slightly higher compared to our study findings. However, the incidence of LTBI, based on positive IGRA results in our study, was considerably higher compared to a previous study conducted among casual contacts in Korea with no identifiable risks of infection (IGRA positivity of < 5% to 10%) [14]. These findings indicate higher risks of LTBI in close contacts of active pulmonary TB patients.
A positive QFT was associated with a positive smear and cough index of the TB source case and a prolonged contact time in studies that involved the evaluation of factors associated with acquiring LTBI in close contacts of active TB patients [17-19]. In addition, residing in the same room as the index case was associated with a positive QFT [20]. However, a positive TST result in close contacts of active TB patients was not associated with a positive smear during the presence of cough and delayed diagnosis [21]. In our previous reports, we showed that there were no significant factors associated with a positive QFT and previous history of TB, and household contact was associated with a positive TST result in close contacts of active TB patients [22].
Approximately 60% of our participants who tested positive for LTBI refused treatment. We believe that the relatively high treatment refusal rate was due to the following factors: (1) participants were reluctant to receive treatment for an infection if they did not experience any symptoms; (2) participants were also concerned about the adverse effects of anti-TB medication; (3) there was no evidence to prove that the infection improved or that the participant was cured; and (4) under the Korean guidelines, treatment was not recommended in participants who were aged > 35 years and had close contact with active TB patients. Interestingly, among the LTBI participants, a higher percentage of women, those with cavitary lesions on the chest radiographs of index cases, and those with positive results for both TST and QFT-G accepted treatment compared to those who refused treatment. Women and those who had cavitary lesions on their chest radiographs were more concerned or anxious about having an infection compared to other participants. Physicians were more prone to diagnose LTBI in participants who had close contact with active TB patients and a positive result for both TST and QFT-G compared to those who had a positive result for only TST or QFT-G. These explanations may lead to a higher probability of accepting LTBI treatment.
Bacilli seem to have low metabolic activity in LTBI and consequently, LTBI should be treated for extended periods in order to be effective. A lengthy treatment regimen along with the asymptomatic nature of LTBI can negatively affect treatment completion [23]. In this study, about half the participants completed their treatment regimen. The acceptance and discontinuation rates of LTBI treatment vary. Only 26% of people identified with LTBI initiated their treatments in a study conducted at public health clinics in the United States; however, only 53% of them completed the treatment course [24]. Horsburgh [8] observed completion rates of 69% and 45% for 4RIF and 9INH among high-risk populations at public clinics. The completion rate was higher for 4RIF compared to 9INH in this study. However, a longer follow-up duration is needed to determine the differences in completion rates because different treatment durations can affect the completion rate of each regimen.
In conclusion, this was the first study regarding the diagnoses and treatments of LTBI in adult close contacts of patients with active pulmonary TB in Korea. The Korean guidelines regarding LTBI treatment should be considered to reduce the incidence of TB among these individuals. Furthermore, studies should also be conducted to verify the efficacy of LTBI treatment and its adverse effects in close contacts of active TB patients.
KEY MESSAGE
1. Among 189 adult close contacts with active pulmonary tuberculosis patients, the positive rate for tuberculin skin test (TST) and QuantiFERON-TB Gold (QFT-G) was approximately 40%.
2. Among 88 participants with latent tuberculosis infection, a high percentage of women, those with cavitary lesions on the chest radiographs of index cases, and those with positive TST and QFT-G accepted treatment.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
The research was funded by the Canadian Institutes of Health Research (NCT00931736).