Five-year clinical outcomes of drug-eluting stents according to on-label and off-label use
Article information
Abstract
Background/Aims:
To compare the clinical outcomes of ‘on-label’ and ‘off-label’ drug-eluting stents (DESs) over a 5-year follow-up period.
Methods:
A total of 929 patients that underwent percutaneous coronary intervention with DESs were enrolled. Patients were divided into two groups according to on-label (n = 449) and off-label (n = 480) indications. Off-label use was defined as implantation of DESs for acute myocardial infarction (MI), very small vessel, a long stenotic lesion, chronic total occlusion, a bifurcation lesion, an ostial lesion, left main coronary artery disease, multivessel disease, a saphenous vein graft lesion, and a lesion with thrombus. Endpoints were composite of major adverse cardiac events (MACEs), which included all-cause death, ischemic-driven target vessel revascularization (Id-TVR), MI, and stent thrombosis (ST). Clinical outcomes in the two groups were compared for up to 5 years postimplantation.
Results:
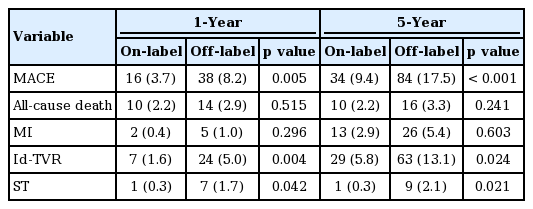
At 1 year postimplantation, the off-label group had higher incidences of total MACEs (8.2% vs. 3.7%, p = 0.005), Id-TVR (5.0% vs. 1.6%, p = 0.004), and ST (1.7% vs. 0.3%, p = 0.042), and at 5 years postimplantation, the off-label group continued to have higher incidences of total MACEs (17.5% vs. 9.4%, p < 0.001), Id-TVR (13.1% vs. 5.8%, p = 0.024), and ST (2.1% vs. 0.3%, p = 0.021). Multivessel disease and diabetes were found to be independent risk factors of MACE in patients with an off-label indication.
Conclusions:
Patients treated with an on-label DES had better long-term clinical outcomes than those treated with an off-label DES.
INTRODUCTION
Drug-eluting stents (DESs) have been widely viewed as the gold standard for the treatment of coronary artery diseases since the Food and Drug Administration (FDA) approved use of the first DES in April 2003. Many randomized trials have reported significant reductions in angiographic restenosis and in the need for revascularization in patients treated with a DES. However, long-term outcomes of DESs with respect to the incidences of late and very late stent thrombosis (ST), which can cause myocardial infarction (MI) and even death, remain debatable [1-4]. For these reasons, the FDA concluded that DES implantation has been demonstrated to be safe for only on-label indications. But, in real-world clinical practice, the applications of DESs have been extended beyond on-label indications, based on the assumptions that benefits extend to more complex cases and lesion subsets [5].
Several studies have been conducted on the clinical outcomes of off-label DES use, but the clinical follow-up data used was limited to 1 year [6-9]. Accordingly, this study was undertaken to compare the clinical outcomes of DESs implanted for on-label and off-label indications over a period of 5 years.
METHODS
Study populations
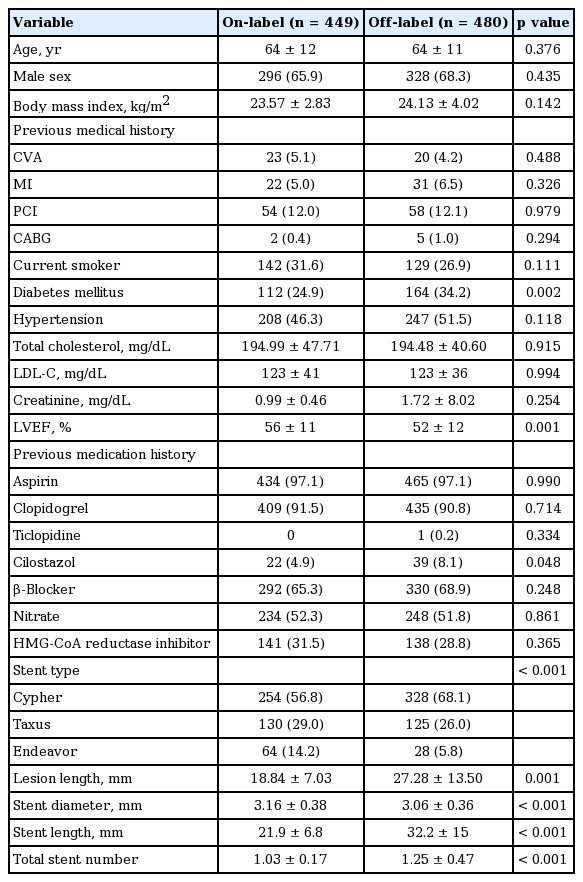
A total of 929 consecutive patients underwent percutaneous coronary intervention (PCI) with a DES from April 2005 to December 2007 at Yeungnam University Medical Center for the treatment of coronary artery disease, and who provided consent were enrolled in this retrospective study. Patients were treated with one of the following DESs; Cypher (sirolimus-eluting coronary stent [SES], Cordis, Warren, NJ, USA), Taxus (paclitaxel-eluting coronary stent [PES], Boston Scientific, Natick, MA, USA) or Endeavor (zotarolimus-eluting coronary stent [ZES], Medtronic, Santa Rosa, CA, USA). On-label indications were for symptomatic ischemic heart disease by de novo lesions ≤ 30 mm long in native coronary arteries of diameter ≥ 2.5 to ≤ 3.5 mm (Cypher), lesions ≤ 28 mm long in arteries of diameter ≥ 2.5 to ≤ 3.75 mm (Taxus), and lesions ≤ 35 mm long in arteries of diameter ≥ 2.25 to ≤ 4.2 mm, including those with diabetes mellitus (Endeavor) [10-12]. Off-label use was defined as DES use, according to the manufacturer’s instructions, for a lesion or patient subset not extensively studied in randomized trials and without FDA approval, and included acute MI (< 48 hours), very small vessel (< 2.5 mm), long stenotic lesions (> 30 mm), chronic total occlusions, bifurcation lesions, ostial lesions (< 5 mm from orifice), left main coronary artery diseases, multivessel diseases, saphenous vein graft lesions, and lesions with thrombus [6,9]. The 929 patients were divided into two groups according to the on-label group (n = 449) and the off-label group (n = 480) indications.
Percutaneous coronary intervention
Decisions regarding the types of DESs used were made by respective operators. Prior to PCI, all patients received a 300 mg of aspirin and 300 or 600 mg of clopidogrel if not already on a maintenance dose or unknown. Unfractionated heparin boluses of 5,000 U were initially administered intravenously and then additional heparin boluses were given to maintain a targeting activated clotting time of 250 to 300 seconds during PCI. Coronary angiography and stenting were performed using routine methods. Daily 100 mg of aspirin and 75 mg of clopidogrel were continued for at least 6 months postimplantation regardless of the stent type. Durations of dual antiplatelet agent administration were determined by physician’s discretion based on the recommendations of the American College of Cardiology/American Heart Association [13].
Outcome parameters and clinical follow-up
Baseline characteristics and laboratory findings were collected. Left ventricular ejection fraction (LVEF) was measured by transthoracic echocardiography during index hospitalization. Procedural data, including the type and number of stents implanted, target lesion sites, and quantitative coronary angiography data (lesion length, stent diameter, and stent length) were assessed by the operator.
The endpoints of this study were composite of major adverse cardiac events (MACEs), which were defined as all-cause deaths, MI, ischemic-driven target vessel revascularization (Id-TVR), and ST. All-cause death was defined as the sum of cardiac and noncardiac deaths, which meant death that was not primarily due to cardiac causes. MI was defined as the presence of at least two of the following: anginal pain, elevation of creatine kinase to more than 3 times the upper limit of normal, and electrocardiographic changes suspicious for MI. Id-TVR was defined as intervention for chest pain or a positive test for ischemia (exercise stress test, stress echocardiogram, resting electrocardiographic evidence of ST-segment deviation in more than 1 lead, or a radionuclide study showing a reversible defect) [14]. ST was classified as ‘definite’ or ‘probable’ as proposed by the Academic Research Consortium, according to which definite ST refers to the presence of thrombus in a stent documented by angiography, and probable ST means any MI related to documented acute ischemia in the territory of an implanted stent without angiographic confirmation, or sudden or unexplained death within 30 days of implantation [15].
Overall clinical outcomes were determined by comprehensively reviewing medical records at routine outpatient visits or by telephone interview during the 5-year follow-up period. A committee independently adjudicated all clinical events. Clinical follow-up data was available for all 929 study subjects.
Statistical analysis
Results were reported as mean ± standard deviation for numerical variables and as frequencies and percentages for categorical variables. The independent t test was used to compare group continuous variables, and the chi-square test or Fisher exact test was used to compare categorical variables. Incidences of MACE, all-cause death, Id-TVR, MI, and ST in the two groups were determined using Kaplan-Meier event curves, and compared using log-rank tests. Multivariate logistic regression analysis was used to identify predictors of MACE in the off-label group. SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis, and statistical significance was accepted for p values < 0.05.
RESULTS
Baseline characteristics including angiographic and procedural data
Of the 929 study subjects, 710 patients were completely monitored for 5 years (follow-up rate, 76.4%). The baseline clinical characteristics of the on- and off-label groups are shown in Table 1. The groups were similar in terms of age, sex, previous medical history, smoking, hypertension, and low density lipoprotein cholesterol level. However, the on-label group had a lower prevalence (24.9% [n = 112] vs. 34.2% [n = 164], p = 0.002) and higher LVEF value (56% ± 11% vs. 52% ± 12%, p = 0.001). More patients in the off-label group were taking cilostazol (8.1% [n = 39] vs. 4.9% [n = 22], p = 0.048), but no significant intergroup difference was found for using of other periprocedural medicines, including other antiplatelet drugs (aspirin, clopidogrel, and ticagrelor), β-blocker, nitrate, or 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor.
Four hundred eighty patients (51.6%) were treated using off-label indications (Table 2) and 22.1% of the patients had more than two off-label indications. The most two common off-label indications were acute MI (n = 221, 46.0%) and chronic total occlusion (n = 381, 20.6%). Stent types and sites of implantation are listed in Table 1. In terms of procedural data, marked differences were observed between the two groups; the off-label group had a longer mean lesion length, a larger mean stent length, a smaller mean stent diameter, and more stents. These differences seemed to be natural consequences of following off-label indications.
One- and five-year outcomes
Over the first year postimplantation; total MACEs (8.2% [n = 38] vs. 3.7% [n = 16], p = 0.005), Id-TVR (5.0% [n = 24] vs. 1.6% [n = 7], p = 0.004), and ST (1.7% [n = 7] vs. 0.3% [n = 1], p = 0.042) were significantly higher in the off-label group, and rates of all-cause death and MI were non-significantly higher in off-label group. Similar tendencies were observed over the 5-year postimplantation period; total MACEs (17.5% [n = 84] vs. 9.4% [n = 34], p < 0.001), Id-TLR (13.1% [n = 63] vs. 5.8% [n = 29]), and ST (2.1% [n = 9] vs. 0.3% [n = 1], p = 0.021) (Table 3).
MACE-free survival curves for the two groups at 1 year and at 5 years are shown in Fig. 1. For both periods, the on-label group had significantly higher event-free survival rates (p = 0.001). Multivariable analysis showed that multivessel disease (hazard ratio [HR], 2.00; 95% confidence interval [CI], 1.09 to 2.49; p = 0.004) and diabetes (HR, 1.65; 95% CI, 1.25 to 3.22; p = 0.017) were significant risk factors of long-term MACE in the off-label group (Table 4).

Major adverse cardiac event (MACE) free survival curve at (A) 1 year and (B) cumulative 5 years. The on-label group had significantly higher event-free survival rates at 1 year clinical follow-up and cumulative 5 years clinical follow-up as shown in A (p = 0.001) and B (p = 0.001)
DISCUSSION
The results of this study showed that the long-term clinical outcomes of off-label DESs use were worse than those of on-label DESs use. However, over the 1 year and the first 5 years postimplantation MACE, Id-TVR, and ST rates were higher in off-label group. On the other hand, all-cause deaths and the incidences of MI were not significantly different in the two groups, though numerically higher in the off-label group. These cumulative results concur with those of previous studies. Qasim et al. [6] showed patients that underwent off-label DES implantation at a single center between April 2002 and April 2006 had higher rates of TVR (13.2% vs. 24.1%, p = 0.0001) and MACEs (17.6% vs. 28.2%, p = 0.0001). Kotani et al. [16] found off-label use did not increased the rates of death or MI, but that off-label use was associated with higher rates of target lesion revascularization, non-target lesion TVR, and total MACEs (10.3% vs. 5.1%, p = 0.030; 10.1% vs. 4.8%, p = 0.007; 24.0% vs. 18.0%, p = 0.005; respectively).
The Deutsches Drug-Eluting Stent [17] and the EVENT (Evaluation of Drug Eluting Stents and Ischemic Events) Registry [8] were the first to examine the clinical outcomes of off-label DES use. In both of these multicenter trials, it was concluded that off-label DES use is associated with higher rates of adverse events including death (4.3% vs. 2.6% and 3.1% vs. 2.1%, respectively) and MI (2.7% vs. 1.9% and 11% vs. 5.3%, respectively) compared to on-label use. In one single center retrospective study [7], no remarkable differences in MACEs (off-label 2.3% vs. on-label 3.0%) or TVR (off-label 1.3% vs. on-label 1.0%) were found after adjusting for possible confounders.
However, majority of the findings mentioned above were derived from evaluations of first generation DESs, which have since been replaced by new generation DESs in clinical practice. Furthermore, follow-ups were relatively short at less than 2 years. Galasso et al. [18] performed a study using a newer generation DES (Endeavor Resolute, which was designed to improve clinical outcomes in patients with more complex coronary artery diseases) to evaluate the safety and efficacy of its off-label use, and concluded its off-label use was not associated with an increased risk of MACE (9.4% vs. 3.4%, p = 0.13), death (4.9% vs. 0.0%, p = 0.38), or TVR (5.5% vs. 3.4%, p = 0.75) over a mean follow-up of 17 months. Serruys et al. [19] examined performance of the second generation everolimus-eluting stent, and included a large number of off-label indications, and obtained MACE, TVR, death, and MI rates of 8.7%, 4.9%, 1.6%, and 13.5%, respectively, at 1 year. Nevertheless, data on the clinical outcomes of new generation DESs is scant.
Our study included the evaluation of the effect about new generation DES on on-label and off-label indication as well as first generation DES, albeit the number of patients treated with newer generation DESs (Endeavor) was small. In fact, the majority of cardiologists have been choosing newer generation DES for treating coronary artery diseases without regard to on-label and off-label indications in contemporary practice and those clinical outcomes data are encouraging.
In the present study, the incidences of MACE and Id-TVR were found to be higher in the off-label group. However, these differences were probably caused the inclusion of lesions of greater complexity, such as longer lesions, smaller diameter coronary vessels, chronic totally occlusive lesions, and other challenging situations like acute MI, included in the off-label group. Furthermore, it can be assumed that the greater proportion of diabetic patients in the off-label group had adverse effects on 1- and 5-year clinical outcomes. The restenosis rate in diabetic patients treated with DES was almost 40%, and the odds ratio of restenosis associated with DM was 1.61 (95% CI, 1.21 to 2.14; p = 0.004). Furthermore, neointimal hyperplasia is known to be strongly correlated with the occurrence of stent-related cardiovascular events [20].
The present study has several limitations that warrant consideration. First, it was conducted retrospectively in a single center. Second, the number of patients treated with a newer generation DES (Endeavor) was small and the proportions so treated differed in the two groups. Third, we did not record prescribed durations of compliance with antiplatelet drugs, and thus, the effects of compliance on clinical outcome, which may be one of the major determinant of ST and other adverse events, were not considered.
In conclusion, patients treated with DESs, which included SES, PES, and ZES, for on-label indications, achieved better short- and long-term clinical outcomes in terms of MACEs, Id-TVR, and ST over a 5-year follow-up. Furthermore, diabetes and multivessel disease were found to be significant, independent risk factors of long-term MACEs in patients treated with a DES for off-label indications.
KEY MESSAGE
1. Patients treated with an on-label drug-eluting stent (DES) had better long-term clinical outcomes than those treated with an off-label DES.
2. Multivessel disease and diabetes were found to be independent risk factors of major adverse cardiac event in patients with an off-label indication.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by Yeungnam University.