Gemcitabine-induced myositis in a diabetes mellitus patient on hemodialysis
Article information
To the Editor,
Myopathy or myositis in cancer patients can be related with a number of etiologies. Inflammatory myositis is the primary disease entity of concern. The association between cancer and inflammatory myopathies has been widely reported in the literature [1]. On the other hand, there have been studies regarding myopathies caused by cytotoxic chemotherapeutic agents [2]. Gemcitabine, a pyrimidine antimetabolite that inhibits DNA synthesis, is an agent commonly used as standard treatment for pancreatic cancer. Only very few case are published describing myopathy or myositis in patients who received gemcitabine [2]. Here we report a case of a 64-year-old man with pancreatic adenocarcinoma presenting with acute swelling of his right arm and face just after five cycles of gemcitabine infusions.
A 64-year-old male presented with sudden onset of right arm swelling without pain. He was diagnosed with type 2 diabetes mellitus 20 years ago resulting in end-stage renal disease. He had been on hemodialysis since 2007. He was diagnosed with pancreatic adenocarcinoma, peritoneal seeding plus liver metastasis in 2013. Five cycles of gemcitabine (1 g/m2/wk) were infused right after the diagnosis. Two weeks after the 1st cycle of gemcitabine, maculopapular erythematous scaly skin lesions appeared on his upper and lower extremities. The lesions soon after disappeared after applying prednisolone-based ointment. On the same month, he developed diffuse abdominal pain and diarrhea that were resolved with intravenous fluids and antibiotics. Twenty days after the 5th cycle of gemcitabine, he was admitted due to sudden swelling of his face and right forearm. He did not consume alcohol and was an ex-smoker with a smoking history of 30 pack-years. On admission, his blood pressure was 137/62 mmHg, heart rate 80 beats per minute, respiratory rate 20 breathes per minute. The patient was afebrile. His right forearm was firm, tender and erythematous compared with the left side. The white blood cell count was 3.00 × 103/µL (79.9% being neutrophils) and the C-reactive protein level was 1.63 mg/dL. Antinuclear antibody (Ab), anti-Jo-1 Ab and anti-myeloperoxidase Ab as well as blood culture results were all negative. To initially rule out cancer-related thromboembolism or superior vena cava syndrome, upper extremity computed tomography (CT) angiography was performed. The findings were unremarkable. Yet, his forearm swelling became worse along with development of severe tenderness, erythema, and heating sense. His right radial pulse became weaker, serum creatine kinase level increased up to 1,477 U/L. Compartment syndrome was then considered. Two days later, he developed pain and swelling in his right thigh, soon followed by his left. Lower extremity CT demonstrated low attenuated lesions with peripheral enhancement in bilateral rectus femoris muscles. Magnetic resonance imaging (MRI) of his right forearm revealed similar findings (Fig. 1). Muscle biopsy from the right rectus femoris muscle showed severe necrosis of muscle fibers with lymphocytic and neutrophilic infiltrations, fibrinoid necrosis of the vascular walls, and focal vasculitis (Fig. 2). The scheduled 6th cycle of gemcitabine was cancelled. Soon after, without any intervention, the multiple areas of myalgia and soft tissue swelling resolved completely within a week.

T2-weighted (A) and gadolinium-enhanced T1-weighted (B) magnetic resonance (MR) images of the forearm. Flexor digitorum profundus, extensor carpi ulnaris, extensor carpi radialis muscles show swelling and increased signal intensity on T2-weighted (A) MR image. Affected muscles show thick rim enhancement and central nonenhancing area on corresponding gadolinium-enhanced T1-weighted (B) image (arrows). Also there are diffuse edematous changes in the forearm muscles, fascia and subcutaneous tissue.
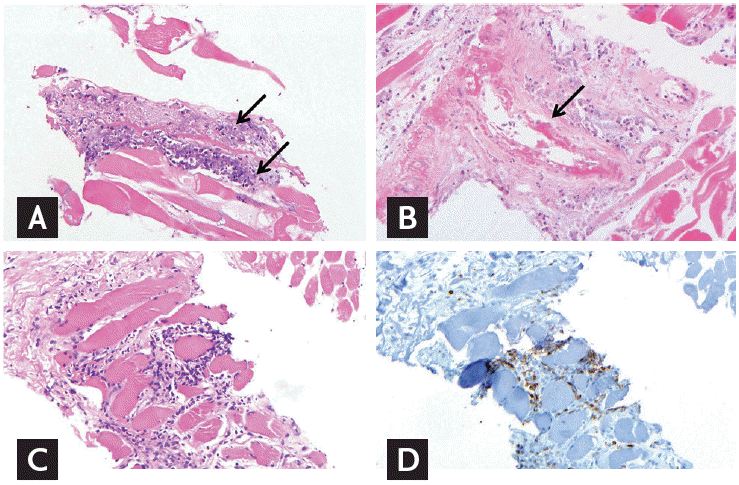
Muscle biopsy shows (A) necrosis of muscle fibers with neutrophilic infiltration (arrows), and (B) fibrinoid necrosis of medium-sized vessel (arrow). (C) There is some lymphocytic infiltration between muscle fibers with mild perimysial fibrosis. (D) Infiltrated lymphocytes are mostly CD3-positive T cells (A, B, C, H&E, ×200; D, immunohistochemistry, ×200).
The most well-known side effect of gemcitabine is radiation recall, which is characterized by muscle lesions and skin rash limited to previously irradiated areas in its users [3]. The mechanism of radiation recall is not yet clearly understood and the known causative drugs include anthracycline (i.e., doxorubicin), taxanes (docetaxel and paclitaxel), and antimetabolites (gemcitabine and capecitabine). Since radiation recall is uncommon and unpredictable, keen awareness is required to aid early diagnosis and appropriate management. Our case, on the contrary, demonstrated that gemcitabine monotherapy even without any prior history of radiotherapy can cause myopathy associated with ischemic injury.
Previous case studies describing myopathy in gemcitabine-treated patients had pancreatic cancer and the lower limbs were affected, especially the thighs as in our case [4]. With cessation of chemotherapy and supportive treatment, symptoms resolved in all cases. MRI, available in one case, showed high signal intensity lesions in T2 fat-saturation images of both thigh muscles, especially in the rectus femoris, as in our patient. Quadriceps muscle biopsy, performed in four patients, showed muscle fiber necrosis and inflammatory infiltrates. Muscle biopsy in our patient specifically demonstrated fibrinoid necrosis in vessel walls, which is a pathologic finding of vasculitis. Spielmann et al. [2] described that the mechanism of muscle injury in gemcitabine may involve vascular mechanism, since thrombosis, endothelial wall thickening, and vascular proliferation were found in biopsied muscle. Gemcitabine can also increase apoptosis of endothelial cells in a dose-dependent manner and enhance cell surface activity of tissue factor [5]. The key differential diagnoses for soft tissue swelling in this case included venous thrombosis, diabetic muscle infarction, inflammatory myopathy. Despite that histopathology in dermatomyositis also incorporates vasculopathy, it is associated with early capillary deposition of the complement C5b-9 membranolytic attack complex [2]. Our case mainly demonstrated fibrinoid necrosis of the medium-sized vessel walls. More importantly, gemcitabine-induced clinical features spontaneously resolve with discontinuation of the agent. Considering the comorbidities described in previous reports and in our case [2], we speculate that the risk of gemcitabine-induced muscle complications may be higher in patients with small vessel disease, observed in long-standing diabetes mellitus and heavy smoking.
To our knowledge, this is the first reported case of gemcitabine-induced myositis in Korea. This case advocates that new onset of musculoskeletal manifestations during gemcitabine use should be carefully evaluated; sudden onset of muscle weakness and swelling could be a presentation of gemcitabine-induced myositis.
Notes
No potential conflict of interest relevant to this article was reported.