Predictive factors of radiographic progression in ankylosing spondylitis
Article information
Abstract
Background/Aims
The course of ankylosing spondylitis (AS) is rather variable, and the factors that predict radiographic progression remain largely obscure. In this study, we tried to determine the clinical factors and laboratory measures that are useful in predicting the radiographic progression of patients with AS.
Methods
In 64 consecutive patients with AS, we collected radiographic and laboratory data over 3 years. Radiographic data included images of the sacroiliac (SI) and hip joints and laboratory data included areas under the curve (AUC) of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), alkaline phosphatase (ALP), and hemoglobin (Hb). We investigated associations among changes in radiographic scores, initial clinical manifestations and laboratory measurements.
Results
Changes in scores for the SI joint and lumbar spine did not correlate with AUC for ESR, CRP, or ALP. AUC for Hb did not significantly correlate with radiographic progression in any joint. Patients with hip arthritis at the initial visit showed significantly higher radiographic score changes after 3 years in the SI and hip joint compared to those without hip arthritis. Patients who had shoulder arthritis as the initial manifestation had significantly increased AUCs for ESR and CRP compared to those without shoulder arthritis. However, at 3 years, the change of the lumbar spine score was significantly higher in patients without shoulder arthritis.
Conclusions
These results indicate that hip arthritis at presentation is a useful clinical marker for predicting the structural damage to the SI and hip joint, and suggest that initial shoulder arthritis correlates with slower radiographic progression of the lumbar spine.
INTRODUCTION
Ankylosing spondylitis (AS) is a common chronic inflammatory rheumatic disease that mainly affects the spine and sacroiliac (SI) joints [1]. Characteristic features of AS include new bone formation, syndesmophytes, and ankylosis of the vertebral column, progressively resulting in significant immobility and functional impairment. Recent research has convincingly shown that radiographic damage in AS interferes with long-term functioning, independent of the actual disease activity [2]; therefore, radiographic damage is an important target for therapeutic intervention in patients with AS [3]. The emphasis in AS management is on early diagnosis and prevention of bony ankylosis.
Along the same lines, predictive markers identifying patients with rapidly progressing disease and poor prognosis would allow tailored treatment. In addition, predictive markers could predict the effect of treatment on radiographic changes.
Because inflammation is an important and powerful element of AS and is considered by most rheumatologists to be linked to ankylosis, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level are frequently used to evaluate patients with AS. Several investigations have searched for predictors of AS disease activity, including acute phase reactants. However, contrary to expectations, only 50% to 70% of patients with active disease have increased levels of CRP and elevated ESRs, indicating a lack of correlation between levels of commonly assayed acute-phase reactants (ESR, CRP) and disease activity [4,5,6,7,8].
Despite the advantages of using clinical or laboratory markers to assess disease activity, which of these measures correlate with disease progression and outcome remains undetermined. Radiographic change is the most concrete marker of disease progression [9], but the factors that predict radiographic progression in AS remain largely obscure.
In this study, we searched for clinical factors and laboratory measures that predict radiographic progression in patients with AS. We scored simple X-rays of the SI and hip joints and lumbar spine and investigated associations among changes in radiographic scores, initial clinical manifestations, and laboratory measurements.
METHODS
Patients
We studied 64 consecutive outpatients who fulfilled the modified New York criteria for AS at Samsung Medical Center, Seoul, South Korea. The study group included 58 males and six females. All patients were taking nonsteroidal anti-inflammatory drugs, and 14 were on corticosteroids (intermittently administered with a daily dose of < 15 mg prednisolone/day). From medical records, we collected information on initial articular symptoms and signs (axial and peripheral joints), disease duration, and history of extra-articular manifestations, including enthesitis and uveitis.
Joint scoring
Plain X-rays of the SI joints (supine anteroposterior films), lumbar spine (anterior/lateral), and hip joints were taken at the time of diagnosis and after 3 years of follow-up. These X-rays were reviewed blindly on two separate occasions by two radiologists. Each SI joint was evaluated according to the modified New York scoring method: 0 (normal), 1 (suspicious change but not definite), 2 (loss of definition, some sclerosis, minimal erosion, some joint space narrowing), 3 (definite sclerosis, blurring, erosive change, loss of joint space), or 4 (ankylosis). Each hip joint was scored by the Larsen method as 0 (normal), 1 (slight abnormality, joint space narrowing, subchondral osteoporosis), 2 (definite early change, erosion, destruction of the joint space of less than 25%), 3 (medium destructive change, destruction of the joint space of 26% to 50%), 4 (severe destructive change, destruction of the joint space of 51% to 75%), or 5 (mutilating abnormality, destruction of the joint space of more than 75%). The lumbar spine was scored by the New Bath Ankylosing Spondylitis Radiology Index method as 0 (normal), 1 (suspicious but not definite), 2 (any number of erosions, squaring, sclerosis with or without syndesmophytes on ≤ 2 vertebrae), 3 (syndesmophytes on ≥ 3 vertebrae), or 4 (fusion involving ≥ 3 vertebrae).
Physical measures
Physical measures included low back pain, back stiffness, spinal mobility, and chest expansion.
Laboratory variables
To estimate the continuous value of each variable over the 3-year period, the areas under the curve (AUC) of ESR, CRP, alkaline phosphatase (ALP), and hemoglobin (Hb) were integrated.
Statistical analysis
Interobserver and intraobserver agreement was analyzed by the κ statistic. Results are presented as means ± SD. Statistical significance between patients with radiological progression and those without was estimated by Student t test. Correlations between variables were analyzed by Spearman test.
RESULTS
Patients had a mean disease duration of 9.25 ± 4.3 years. Fifty-eight of the patients were male, and six were female. Details on clinical and laboratory measures and changes in radiological scores are summarized in Table 1.
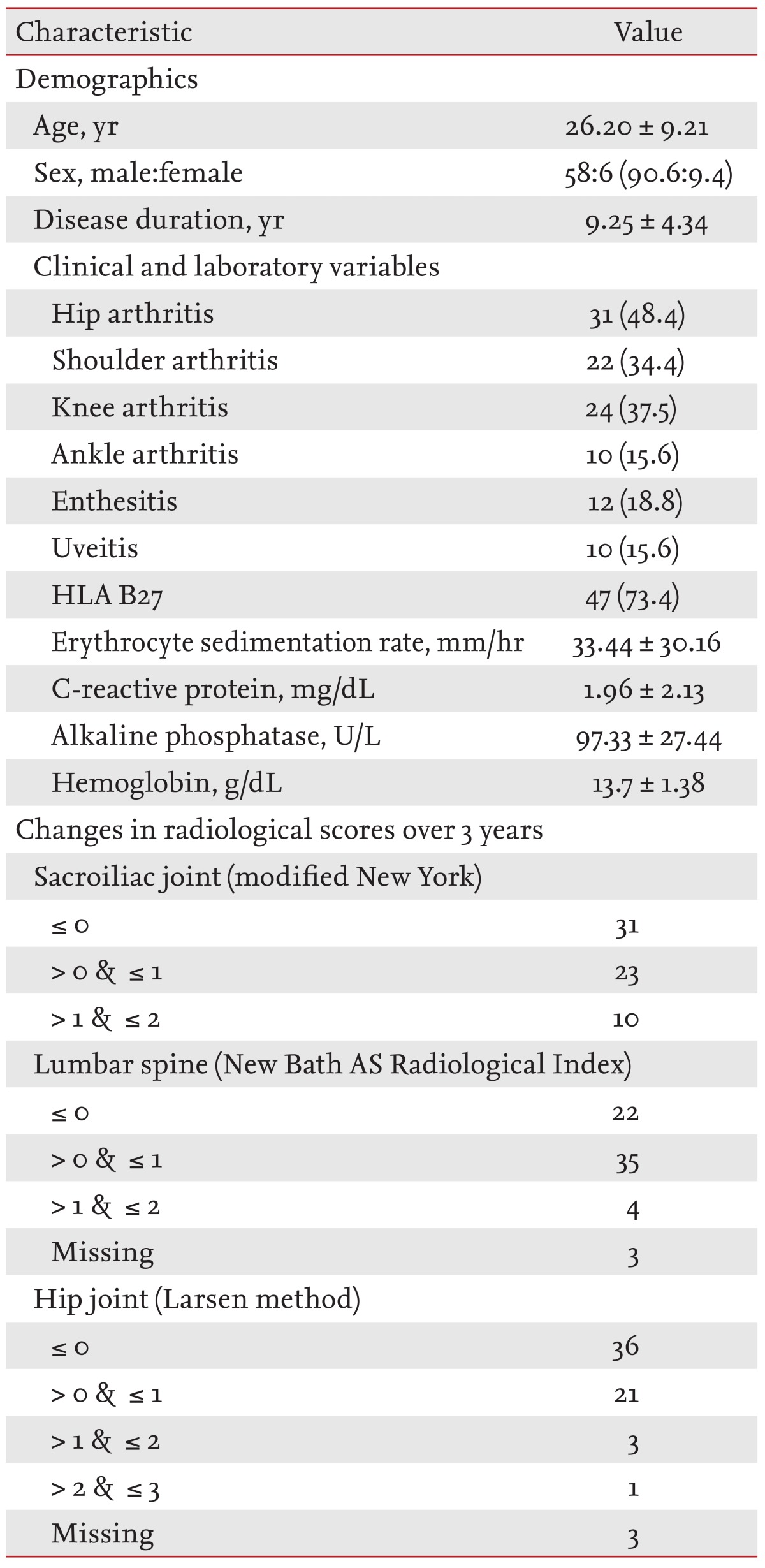
Clinical, laboratory characteristics at diagnosis and radiological changes of ankylosing spondylitis patients (n = 64)
Clinical and laboratory variables
Patient age correlated with ESR (r = 0.355, p = 0.004) but not with CRP. ESR and CRP showed moderate positive correlation (r = 0.524, p < 0.001), and both were moderately negatively correlated with Hb (with ESR, r = -0.513, p < 0.001; with CRP, r = 0.434, p < 0.001). No clinical variables other than age correlated with any other physical or laboratory measures.
Relationships between clinical/laboratory measures and radiological progression
A positive correlation was found between the change in hip joint score and the AUCs for ESR and CRP (r = 0.267, p = 0.038; r = 0.501, p < 0.001, respectively). The correlation of the hip joint score change with CRP was stronger than with ESR. Changes in lumbar spine and SI joint scores did not correlate with any laboratory measures. Radiological progression of the SI and hip joint in patients with hip arthritis as an initial manifestation significantly differed from that in other patients (0.36 ± 0.54 vs. 0.02 ± 0.45, p = 0.019 for SI joint; 0.45 ± 0.78 vs. 0.09 ± 0.32, p = 0.031 for hip joint) (Fig. 1). Patients with shoulder arthritis had significantly increased AUCs for ESR (38.24 ± 27.46 vs. 23.26 ± 25.48, p = 0.048) and CRP (2.979 ± 2.92 vs. 1.31± 1.88, p = 0.016) than those without shoulder arthritis (Fig. 2A and 2B). However, the change in lumbar spine score was significantly higher in patients without shoulder arthritis as an initial manifestation than in those with initial shoulder arthritis (1.64 ± 4.21 vs. 6.23 ± 7.56, p = 0.030) (Fig. 2C). On the other hand, change in SI and hip joint scores was not associated with shoulder arthritis (p = 0.284 for SI joint; p = 0.165 for hip joint). Changes in lumbar spine score did not significantly differ between those with hip arthritis and those without (4.4 ± 7.06 vs. 4.19 ± 6.59, p = 0.030).

Change in radiographic score over 3 years in the (A) sacroiliac (SI) joints and (B) hip joints in patients with initial hip arthritis versus those without.

Comparison of patients with shoulder arthritis as initial articular manifestation to those without. (A, B) areas under the curve (AUC) for (A) erythrocyte sedimentation rate (ESR) and (B) C-reactive protein (CRP); (C) change in lumbar spine score. LS, lumbar spine; BASRI, Bath Ankylosing Spondylitis Radiology Index.
DISCUSSION
Structural damage is an important outcome in many rheumatic diseases with arthritis, and AS is no exception [10]. However, the progression of joint damage in AS is highly variable and unpredictable. In some cases, radiographic progression is very slow; in other cases, extensive or rapid bony ankylosis occurs within a few years after disease onset. Therefore, distinguishing patients with more aggressive disease courses from those likely to have slower progression by predictive markers would help in selecting appropriate treatment and assessing response to therapy. Given that there is no gold standard available for measuring disease activity or predicting disease course and radiological outcome in AS, many factors have been investigated. Acute phase reactants, such as ESR and CRP, are considered objective indicators of inflammation [8]. In RA, numerous studies have suggested that high ESR and CRP levels at onset or during the disease course independently predict longterm radiographic progression [11]. In AS, however, their value in determining disease activity is rather limited; elevated ESR and CRP are not frequently present in AS and do not correlate well with clinical activity or radiological progression [4,5,6,7,9]. In our study, we also found no correlation among the AUCs of ESR, CRP, and progression in axial joints (SI and lumbar spine). However, changes in hip joint scores correlated with AUC for ESR and CRP. This result is in accordance with previous studies, which have shown that both acute-phase reactants and Bath AS Disease Activity Index scores are higher in patients with peripheral joint involvement than in patients with purely axial disease [4,12,13,14]. We also observed significant radiological progression in the SI and hip joints in patients with hip arthritis at presentation, suggesting that early hip arthritis may predict radiological progression in the hip joints, while acute phase reactants, such as ESR and CRP, reflect arthritis activity in the hip joints. Because Amor et al. [15] reported that hip involvement in spondyloarthropathies predicts severe outcome, we anticipated this result. In this study, however, no significant association between hip arthritis and structural progression in the lumbar spine was found.
On the other hand, structural damage progression in the lumbar spines was significantly slower in patients with shoulder arthritis at presentation, suggesting that peripheral joint involvement in AS may predict less severe structural damage in the spine. This is consistent with a recent study by Baek et al. [16], which reported less radiographic change in patients with peripheral joint arthritis. On the contrary, Carette et al. [17] noted previously that early involvement of peripheral joints correlates with poorer prognosis for spinal mobility. Hips and shoulders are the peripheral joints most commonly affected in patients with AS. Hip involvement has been recognized as a common and disabling problem, whereas shoulder involvement is less frequent and less severe [18]. This correlation of hip arthritis with disease progression in AS may result from the fact that the hip joints are anatomically closer to the axial skeleton. However, histopathology indicates that AS in the hip involves inflammation and erosion of the subchondral bone marrow [19], not formation of new bones, in contrast to the classical changes caused by AS in the spine. Given previous conflicting findings, our results are not sufficient to indicate that peripheral arthritis is a protective predictor against structural damage in the spine. Because little information is available on the association of peripheral arthritis and radiographic progression in the spine in AS, further study is required to clarify this issue.
Anemia of chronic disorders is an indicator of disease activity in systemic autoimmune disease [20,21], thought to be mediated by inflammation-associated cytokines [22]. We investigated, therefore, the relationship between AUCs for Hb levels with those for ESR and CRP and changes in radiographic scores, and found that Hb correlated negatively with ESR and CRP but did not relate to radiographic changes. This result suggests that systemic inflammation does not correlate with progression of bony ankylosis.
We also evaluated whether levels of the bone formation marker ALP correlate with radiographic changes and found no relationship. Several studies have found significant relationships between acute-phase reactants and biochemical markers of bone resorption, such as urinary pyridinoline, f-Pyr, f-Dpyr, and collagen pyridinium cross-links [23,24,25]. Thus, bone resorption markers may be more likely to relate to AS progression.
The lack of correlation between radiographic progression, in particular ankylosis in the spine, and serological markers of inflammation may be explained by the observation that the biomarkers measured primarily reflect peripheral synovitis rather than the ankylosing process in the axial joints. This observation supports the idea that the dominant feature reflecting structural damage in AS is osteoproliferation rather than bone erosion. A recent study demonstrated that inflammation and joint remodeling are uncoupled in a mouse model of spondyloarthritis, which reinforces this hypothesis [26]. However, the interactions of inflammation and new bone formation in AS deserve further study.
This study has several potential limitations, including its relatively small sample size. This was not a prospective study, but its longitudinal nature makes it better suited to evaluate the impact of fluctuating inflammation on structural damage than a cross-sectional study. All of patients in this study were TNF-a inhibitor naive because TNF-a blockers could reduce inflammation effectively in AS patients [27,28]. An analysis over a longer period of observation would be desirable; however, our assessment period was longer than the minimum required for significant changes in new bone formation (2 years). The modified Stoke Ankylosing Spondylitis Spine Score might have been a better choice for our purposes because it focuses heavily on osteoproliferation and is considered superior for evaluation of structural changes in the spine [29,30]. Nonetheless, all scoring methods used in this study to assess the structural damage of patients with AS were validated methods of evaluation.
This study suggests that hip arthritis at the time of diagnosis is a useful clinical predictor of radiographic progression in the SI and hip joints. On the other hand, initial shoulder arthritis seems to correlate with slower radiographic progression in the lumbar spine.
Our study's aim was to identify reliable and predictive factors for radiographic progression in AS. Given the consensus that systemic inflammation is likely to relate to radiological progression in AS, a number of studies have focused on the relationship between clinical disease activity and inflammatory markers. Despite the limitations of the present study, such as its small sample size and retrospective design, these results could help us understand the factors that predict bony structural changes in patients with AS.
KEY MESSAGE
The inflammatory markers erythrocyte sedimentation rate and C-reactive protein are not associated with radiographic progression of the lumbar spine in ankylosing spondylitis (AS).
Hip arthritis at presentation is a useful predictor of structural damage to the sacroiliac and hip joints in AS.
Peripheral arthritis, especially shoulder arthritis, might predict slower radiographic progression of the lumbar spine in AS.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.