The relationship between serum asymmetric dimethylarginine levels and subjective sleep quality in normotensive patients with type 2 diabetes mellitus
Article information
Abstract
Background/Aims
Poor sleep quality (SQ) is associated with increased cardiovascular mortality and morbidity. Additionally, asymmetric dimethylarginine (ADMA) is an independent predictor of cardiovascular mortality and morbidity. However, no sufficient data regarding the relationship between ADMA levels and SQ have been reported. The goal of the current study was to evaluate the association between SQ and ADMA levels in normotensive patients with type 2 diabetes mellitus.
Methods
The study participants consisted of 78 normotensive type 2 diabetics. The SQ of all participants was assessed using the Pittsburgh Sleep Quality Index (PSQI). Patients with a global PSQI score > 5 were defined as "poor sleepers." Factors associated with poor SQ were analyzed using a multiple regression model. Serum ADMA levels were measured using high performance liquid chromatography.
Results
The median ADMA levels of the poor sleepers were increased compared with patients defined as good sleepers (5.5 [4.2 to 6.6] vs. 4.4 [2.9 to 5.4], p < 0.01, respectively). However, the L-arginine/ADMA ratio was decreased in poor sleepers (p < 0.01). Global PSQI scores were positively correlated with ADMA levels (p < 0.01) and negatively correlated with the L-arginine/ADMA ratio (p = 0.02). ADMA levels were correlated with sleep latency (p < 0.01) and sleep efficiency (p = 0.01). Logistic regression analysis showed that ADMA levels (odds ratio [OR], 1.68; 95% confidence interval [CI], 1.16 to 2.44; p = 0.01) and body mass index (OR, 1.15; 95% CI, 1.01 to 1.31; p = 0.04) were associated with poor SQ independently of glomerular filtration rate, sex, age, duration of diabetes, hemoglobin A1c, total cholesterol, and systolic blood pressure.
Conclusions
Self-reported SQ was independently associated with ADMA levels in normotensive patients with diabetes mellitus.
INTRODUCTION
Sleep quality (SQ) is an important factor in the quality of life. Many organic diseases and sleep disorders impair SQ in different ways. Polysomnography is the best method to objectively assess SQ. However, many studies have shown that self-reported SQ also gives abundant information about a patient's prognosis [1,2,3]. Subjective SQ, that is self-reported SQ, is commonly assessed using the Pittsburgh Sleep Quality Index (PSQI) scale and can be translated as the individual's perceived SQ. It is often ignored in daily clinical practice. A poor SQ may be the forerunner of the development of coronary artery disease, hypertension, and diabetes mellitus. Moreover, poor SQ is associated with increased cardiovascular mortality and morbidity [4]. Several studies have demonstrated that poor SQ is increased 3- to 4-fold and cardiovascular disease 2- to 3-fold in individuals with diabetes mellitus compared with those without diabetes mellitus [5]. An organic basis for this relationship has not yet been clearly demonstrated.
Several studies in the literature have indicated that increased levels of inflammatory markers such as high-sensitivity C-reactive protein, interleukin 6 (IL-6), tumor necrosis factor α (TNF-α), and E-selectin are related to poor SQ in the general population [6,7,8]. Elevated inflammatory markers, such as IL-6 and soluble intercellular adhesion molecule, were associated with an increased morbidity and mortality in apparently healthy men, as reported by Ridker et al. [9]. The relationship between inflammation and poor SQ has been reported in various studies [6,7,8]. The association of self-reported SQ and sleep duration with IL-6, IL-1β, and TNF-α levels among a community sample of 156 healthy adults was examined, and it was determined that poor SQ is associated with a greater production of all three of these inflammatory mediators. Pro-oxidant and proinflammatory conditions inhibit the activity of dimethylarginine dimethylaminohydrolase, which metabolizes asymmetric dimethylarginine (ADMA). It has been demonstrated that ADMA plasma levels are elevated in subclinical inflammation states, including patients with multiple risk factors for atherosclerosis. Therefore, we hypothesized that poor SQ may be related to increased ADMA levels.
The current studies have focused on the above-mentioned question. However, diabetes mellitus is an important contributor to both poor SQ and cardiovascular mortality. ADMA, an endogenous nitric oxide inhibitor, is commonly increased among patients with type 2 diabetes mellitus [10], and it is an independent predictor of cardiovascular mortality and morbidity in patients with diabetes mellitus [11]. However, the relationship between SQ and ADMA among patients with type 2 diabetes mellitus has yet to be investigated. Therefore, our goal was to investigate the relationship between SQ and ADMA levels among patients with type 2 diabetes mellitus.
METHODS
Patient selection
In this cross-sectional study, 78 normotensive patients with type 2 diabetes mellitus (male:female, 30:48) were enrolled. Patients who presented to the endocrinology outpatient clinic for routine glycemic control were selected for the study. All patients included in this study had been diagnosed with type 2 diabetes mellitus for a minimum of 6 months prior to enrolling in the study. This study was approved by the local Ethics Committee and adhered to the Declaration of Helsinki. Written informed consent was obtained from all patients participating in the study.
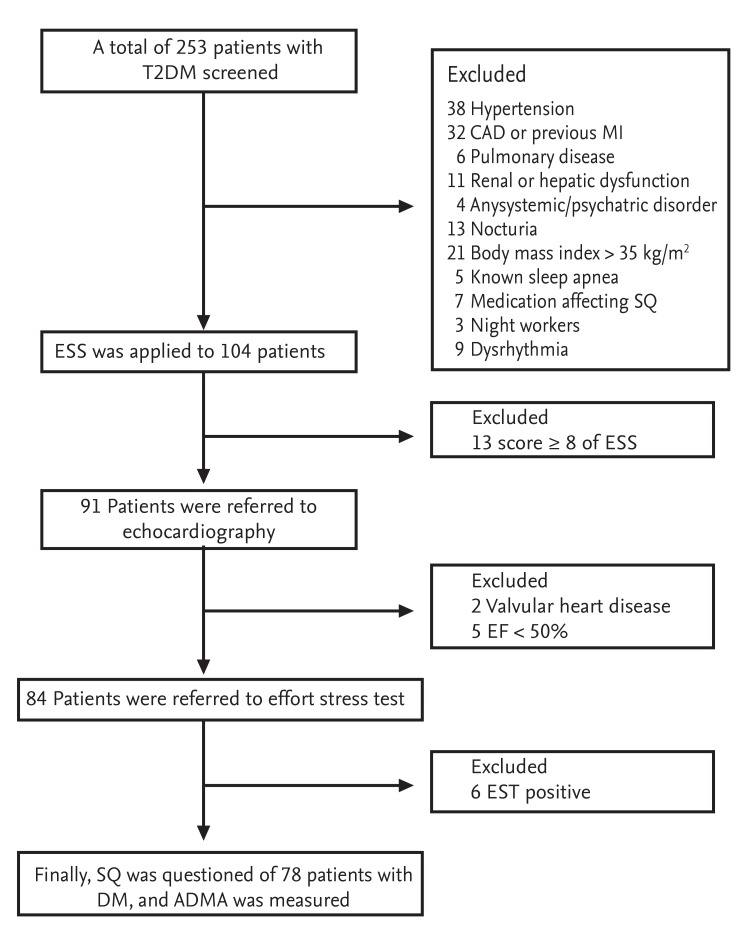
Exclusion criteria included patients with any of the following: hypertension, antihypertensive medication use, coronary artery disease, previous myocardial infarction, revascularization history, valvular heart disease, left ventricular segmental wall motion abnormality, ejection fraction below 50%, dysrhythmias such as atrial fibrillation, pulmonary disease, renal or hepatic dysfunction, any systemic disorder, nocturia, severe obesity (body mass index [BMI] > 35), known sleep apnea, a score ≥ 8 on the Epworth Sleepiness Scale, psychiatric medication use, alcohol abuse, and an evening occupation, such as security guards and long-distance drivers. Patients on hypnotics or any medications directly affecting SQ were also excluded from the study. Cases of silent myocardial ischemia, as diagnosed by a treadmill stress test, were excluded. A positive stress test was defined as a horizontal and/or down-sloping ST segment depression above 1 mm in two consecutive leads. A flow-chart diagram of the study population is shown in Fig. 1.
Study protocol
Type 2 diabetes mellitus subjects were questioned regarding a history of hypertension. Blood pressure (BP) was measured twice in the office, with a 10-minute resting interval in between, using a mercury sphygmomanometer (ERKA D-83646 Bad Tolz, Kallmeyer Medizintechnik GmbP Co. KG, Bad Tolz, Germany). Patients with a BP < 140/90 mmHg, who were not taking any antihypertensive medications, were asked to evaluate their quality of sleep using the PSQI questionnaire. After a 10-hour fast, blood samples were obtained from all patients in the morning the following day between 9:00 and 11:00 AM. Blood samples were stored at -80℃, and ADMA and L-arginine were measured in these samples at the end of the study using high performance liquid chromatography. Other routine biochemical tests, including a complete blood count and metabolic panel, were analyzed on the same day as the blood draw.
The interassay coefficient of variation (CV) of ADMA was calculated as 6.6%, the intra-assay CV as 4.7%, and the imprecision of the assay as < 10%. Additionally, the CV values of L-arginine were evaluated. The inter-assay CV of L-arginine was calculated as 7.0%, the intra-assay CV as 5.5%, and the imprecision of the assay as < 10%. All tests were performed in duplicate.
Sleep assessment
SQ was measured using the PSQI [12]. This self-reported questionnaire assesses SQ and contains 19 self-rated questions that aid in differentiating SQ. The PSQI is comprised of seven components: subjective SQ, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime drowsiness and dysfunction. Each component is scored from 0 to 3, yielding a global PSQI score between 0 and 21. Higher scores indicate a lower SQ. A global PSQI score greater than 5 has an 89.6% sensitivity and an 86.5% specificity in identifying patients with poor versus good SQ [12].
Blood sample analyses
For ADMA analysis, peripheral venous blood samples (5 cc) were collected in Vacutainer tubes without anticoagulant. The tubes were centrifuged immediately at 3,000 ×g for 10 minutes. The separated serum samples were stored at -80℃. Measurement of ADMA was accomplished using high performance liquid chromatography (HP Agilent 1200, Agilent Technologies, Palo Alto, CA, USA), with modification of the method described by Chen et al. [13]. GFR was calculated from serum creatinine measurements using the Cockcroft-Gault formula [14].
Statistical analyses
All data were analyzed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). The distribution of variables was analyzed using the Kolmogorov-Smirnow test. Normally distributed data are presented as means ± standard deviations. Data with abnormal distributions are expressed as medians (interquartile range), and dichotomous data are presented as percentages. The significance level of the differences between two groups was assessed using independent Student t tests for normally distributed variables and the non-parametric Mann Whitney U test for non-normally distributed variables. The differences between the categorical variables were determined using the chi-square test. Linear association between parametric variables was evaluated using Pearson correlation test. Correlation analysis of non-parametric data was performed using the Spearman test. Stepwise multivariate logistic regression was analyzed to determine the factors associated with poor SQ (PSQI > 5). The level of statistical significance was accepted as p < 0.05 for all tests.
Power analysis was performed using the Minitab 16 packet program. The sample size was calculated as 70 to determine a difference in ADMA of approximately 1.0 µmol/L with 80% power and 95% confidence interval (CI). The study sample was enlarged by approximately 10% to counteract a potential loss of data during the blood sample analysis. A MedCalc 9.2.0.1 packet program was used to calculate receiver operating characteristic curves and to analyze specificity, sensitivity, negative, and positive predictive values of ADMA on poor SQ.
RESULTS
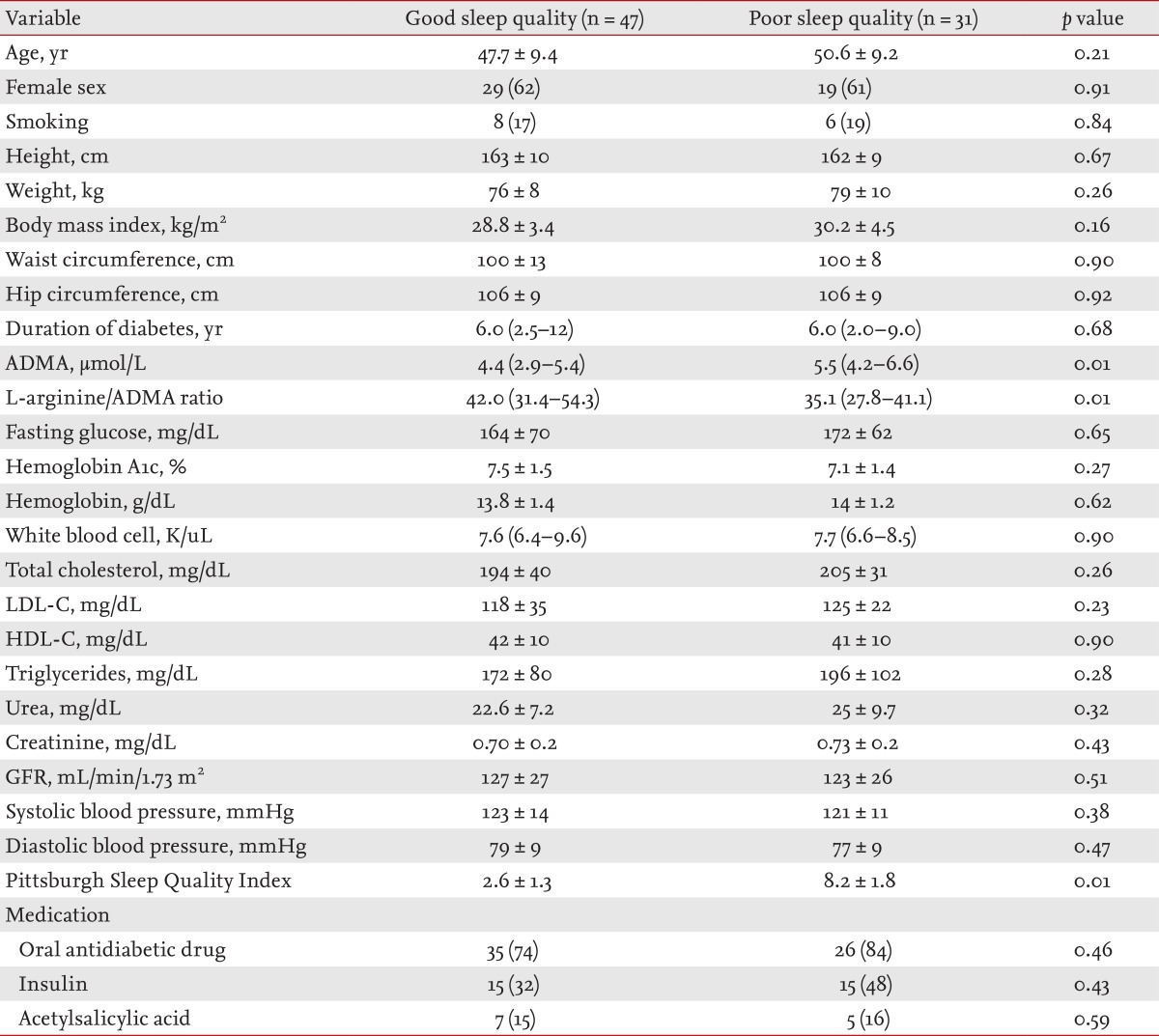
The baseline characteristics of the poor SQ and good SQ groups are shown in Table 1. ADMA levels of the poor sleepers were increased compared with the good sleepers (p < 0.01). In addition, the L-arginine/ADMA ratio was lower in poor sleepers than in good sleepers (p < 0.01). Other parameters, such as duration of type 2 diabetes mellitus diagnosis, hemoglobin A1c (HbA1c) and other laboratory findings, were comparable between the two groups (Table 1).
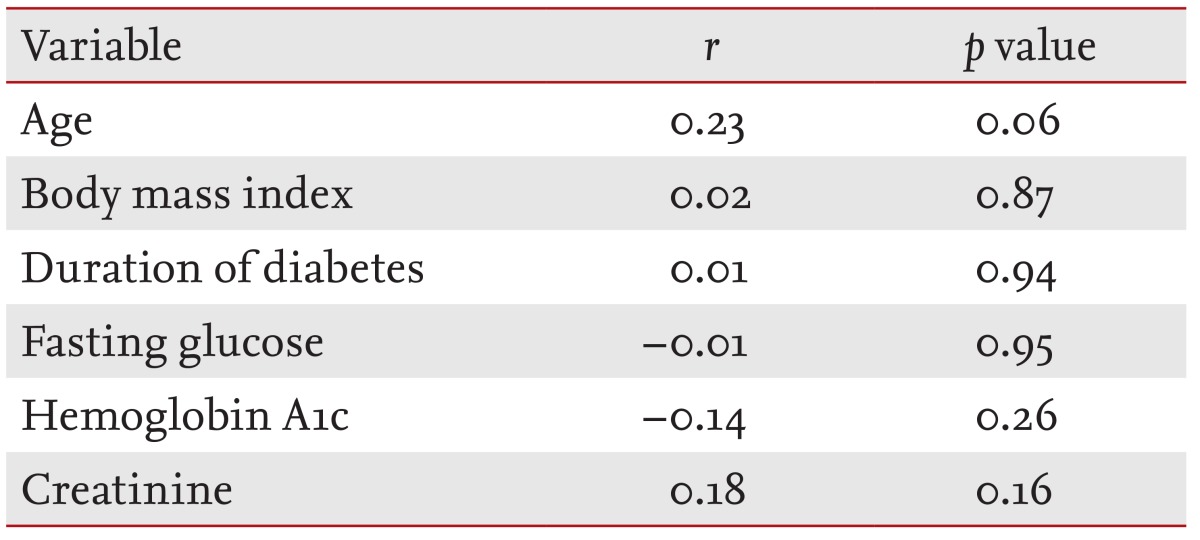
When linear associations were evaluated, it was determined that the global PSQI was correlated with ADMA levels (p < 0.01) (Fig. 2) and inversely correlated with the L-arginine/ADMA ratio (p = 0.02) (Table 2). ADMA levels were also correlated with both sleep latency (p < 0.01) and sleep efficiency (p < 0.01) (Table 2). Similarly, the L-arginine/ADMA ratio was inversely correlated with sleep latency (p < 0.01), the global PSQI, and sleep efficiency (p = 0.02) (Table 2). The global PSQI was not correlated with demographics or other laboratory findings (Table 3).
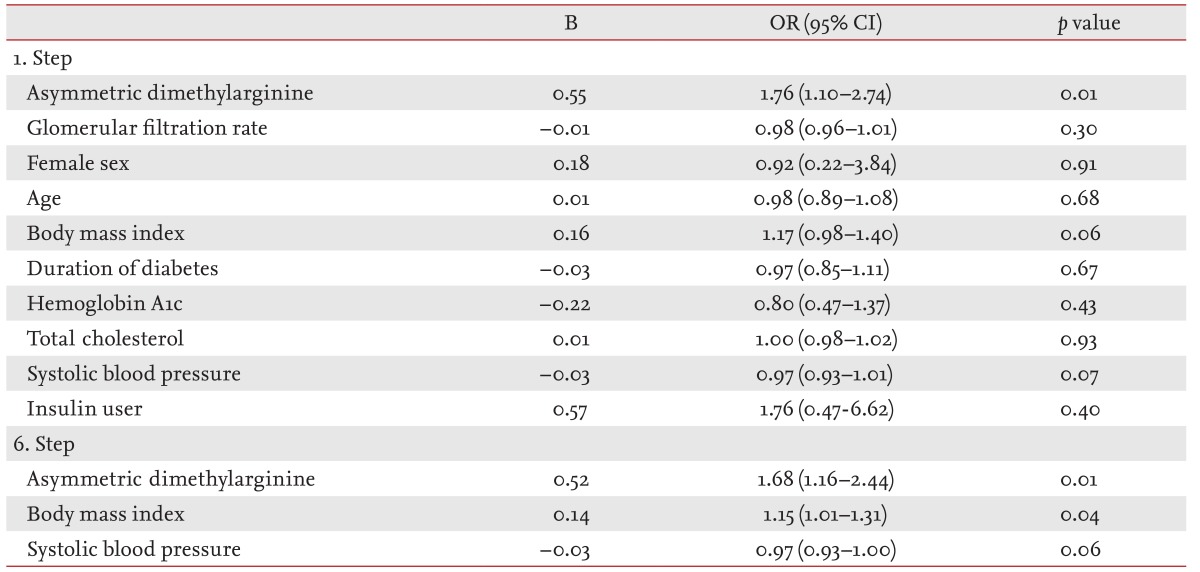
In stepwise multivariate logistic regression analysis, the ADMA level was associated with poor SQ (odds ratio [OR], 1.68; 95% CI, 1.16 to 2.44; p = 0.01), independently of GFR, sex, age, BMI, duration of diabetes, HbA1c, total cholesterol, and systolic BP. Additionally, body mass index was also associated with poor SQ (OR, 1.15; 95% CI, 1.01 to 1.31; p = 0.04) (Table 4). However, ADMA was not correlated with BMI (r = -0.17, p = 0.17).
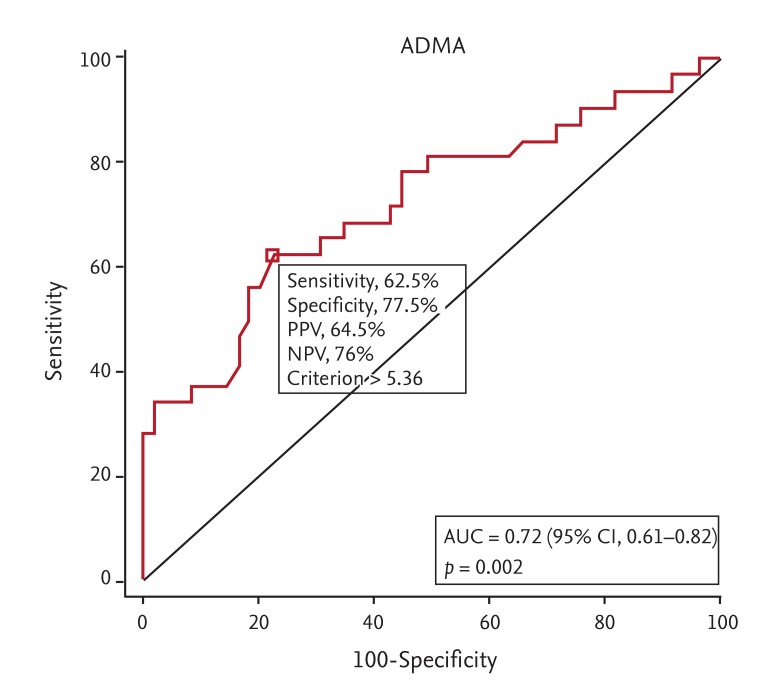
A cut-off value was examined to determine the positive predictive value for poor SQ. The optimal cutoff value was determined to be > 5.36 µmol/L ADMA using the MedCalc 9.2.01 program, and the area under the curve was found to be 0.72 (standard error = 0.060, p < 0.01) for this cutoff value. The following results were obtained using the cutoff value > 5.36 µmol/L ADMA: sensitivity, 62.5%; specificity, 77.5%; positive predictive value, 64.5%; and negative predictive value, 76% (Fig. 3).
DISCUSSION
We determined that serum ADMA levels were significantly increased in patients considered poor sleepers, and that ADMA levels can serve as an independent factor associated with poor SQ in normotensive patients with type 2 diabetes mellitus. Linear relationships between ADMA levels and SQ parameters were also observed. To the best of our knowledge, this is the first study to examine the association between self-reported SQ and ADMA levels in this population.
There are conflicting results about the relationship between poor SQ and glycemic control in patients with type 2 diabetes mellitus [3,15]. Knutson et al. [15] reported that lower SQ is associated with poorer glucose control after controlling for age, sex, BMI, insulin use, and the presence of major complications. Also, Tsai et al. [3] showed a strong positive association between poor SQ and HbA1c level after adjusting for a large number of possible confounders. We did not observe any relation between ADMA levels and HbA1c. Mean HbA1c values were above 8% in the aforementioned studies, while they were approximately 7% in the current study. Therefore, relatively good glycemic control may explain our particular finding regarding the relationship between HbA1c and SQ. Similarly, we also did not detect a relationship between HbA1c and ADMA in the present study. The relationship between ADMA levels and glycemic control has exhibited controversial results in the literature [3,15,16,17,18]. Can et al. [16] showed that patients with type 2 diabetes mellitus and poor glycemic control had higher ADMA concentrations than patients with well-controlled glycemia. In contrast, Paiva et al. [17] reported that ADMA levels were inversely related to HbA1c levels in patients with type 2 diabetes mellitus due to renal hyperfiltration. Also, Marcovecchio et al. [18] showed a negative association between HbA1c and ADMA in young people with type 1 diabetes due to the down-regulation of ADMA production or stimulation of its metabolism. As a result, HbA1c levels were not related to either ADMA levels or SQ.
SQ is also associated with obesity [19]. Hung et al. [19] demonstrated that female gender, being overweight, obesity, and sleep duration were associated with poor SQ independently of cardiometabolic risk factors. It has been recommended that subjects who are obese or overweight be evaluated for the presence of sleep disturbance [19]. We found BMI to be an independent factor associated with poor SQ (OR, 1.15; 95% CI, 1.01 to 1.31; p = 0.04) in logistic regression analysis. Despite exclusion of severely obese individuals with a BMI > 35 kg/m2, BMI remained associated with poor SQ. ADMA levels are increased in obesity [20], yet, we did not find a linear association between serum ADMA levels and BMI. Koc et al. [20] reported that ADMA levels were significantly higher in obese individuals compared with healthy controls. In the study by Koc et al. [20], the mean BMI was 35.0 ± 3.3 in the obese group, while in the current study, it was 29.4 ± 3.5. Considering that severely obese subjects were excluded from the present study, it is likely that an obvious relationship between BMI and ADMA has been diminished. Although BMI was not correlated with ADMA levels, BMI was still determined to be an independent factor associated with poor SQ in the current study. One possible explanation for this is that BMI may utilize an ADMA-independent pathway in the impairment of SQ.
Although no significant observation in regards to a relationship with BP and SQ was found between the two groups, a trend was observed demonstrating an inverse relationship with BP and SQ using logistic regression analysis. This suggests that a lower BP in type 2 diabetes mellitus patients does not enhance SQ. Subjective SQ deteriorates with aging; however, there is also an increase in the incidence of diseases, such as atherosclerotic heart disease, and hypertension, with aging [21]. Vitiello et al. [21] showed that asymptomatic older adults manifested a significantly greater amount of disturbed sleep compared with healthy, younger subjects. We also observed an association between increasing age and poor SQ in the present study (p = 0.06). However, while the mean age of the patients in the study of Vitiello et al. [21] was 67.5 ± 0.5 years, the mean age of the patients in the current study was approximately 50 years. Therefore, we believe that a possible relationship between age and poor SQ may be masked by the relatively younger age of the patients in the present study.
The pathophysiological mechanisms of the association between poor SQ and poor cardiovascular outcome have not been fully elucidated in patients without overt obstructive sleep apnea syndrome. There are some reports citing a relationship between sympathetic hyperactivation and poor SQ [22,23]. Poor SQ may lead to sympathetic hyperactivation via disturbances in the normal circadian variation of the autonomic nervous system. Additionally, there are some data suggesting that sympathetic neural activation and ADMA may affect the level of each other [24,25]. As seen in the aforementioned studies, both ADMA levels and SQ have been individually linked to sympathetic hyperactivation, suggesting that the potential mechanism contributing to the association between poor SQ and serum ADMA levels may be the sympathetic hyperactivation induced by poor SQ [4,5,21,24,25]. However, we did not examine sympathetic activation in the current study, which may contribute to the mechanisms behind serum ADMA levels and poor SQ relationship.
Limitations
An important limitation of the present study was the unavailability of polysomnographic data. Although a strong correlation between actigraphy and subjective assessment was shown with changes in sleep patterns in the study by Lockley et al. [26], there are some discrepancies between the two methods. Additionally, ADMA levels are high in patients with obstructive sleep apnea syndrome, and the subjects in this study were not evaluated for the presence of obstructive sleep apnea syndrome. However, the Epworth Sleepiness Scale was performed on all patients, and this scale is often used clinically to screen for manifestations of the behavioral morbidity associated with obstructive sleep apnea syndrome. Rosenthal and Dolan [27] reported that an Epworth Sleepiness Scale score of 10 has a 66% sensitivity and a 48% specificity, and a score of 8 on the Epworth Sleepiness Scale has a 76% sensitivity and a 31% specificity for obstructive sleep apnea syndrome with an apnea-hypopnea index of ≥ 5. Therefore, we chose 8 as our Epworth Sleepiness Scale cutoff score for excluding patients from the present study. Using this method, our goal was to exclude obstructive sleep apnea syndrome patients from the study. Another limitation is that one of the probable causes of sleep problems is depression, and the level of depression was not measured in our study. Effort stress tests were performed in all patients to exclude any unknown coronary artery disease. However, since the sensitivity and specificity of an effort stress test varies from 45% to 67% and from 72% to 90% respectively [28], some patients with coronary artery disease may have not been detected. Other markers of inflammation, leptin, and the sympathetic activation parameters and their relationships with ADMA could not be evaluated due to a limited budget. However, we chose to perform ADMA measurements because the American College of Cardiology/American Heart Association guidelines advise the use of ADMA levels for screening CV risk in asymptomatic diabetic subjects. Thus, ADMA is a well-studied and accepted biomarker for CV risk screening in diabetic subjects. Lastly, the sample size of the study was relatively small, but the power of the study was determined to be acceptable.
KEY MESSAGE
A possible link between asymmetric dimethylarginine levels and poor sleep quality (SQ) in patients with type 2 diabetes mellitus.
It provides insight into the mechanism(s) by which poor SQ and adverse health outcomes occur in patients with type 2 diabetes mellitus.
Acknowledgments
This project was supported by Selcuk University Scientific Research Projects Fund.
Notes
Conflict of interest: No potential conflict of interest relevant to this article was reported.