Complex repetitive discharge on electromyography as a risk factor for malignancy in idiopathic inflammatory myopathy
Article information
Abstract
Background/Aims
We investigated the electromyography (EMG) findings and demographic, clinical, and laboratory features that may predict the development of malignancy in patients with idiopathic inflammatory myopathy (IIM).
Methods
In total, 61 patients, 36 with dermatomyositis and 25 with polymyositis, were included. Patients were divided into those with and without malignancies, and comparisons were made between the groups in terms of their demographic, clinical, laboratory, and EMG findings.
Results
The frequencies of malignancies associated with dermatomyositis and polymyositis were 22% and 8%, respectively. Patients with malignancies showed a significantly higher incidence of dysphagia (odds ratio [OR], 21.50; 95% confidence interval [CI], 3.84 to 120.49), absence of interstitial lung disease (ILD; OR, 0.12; 95% CI, 0.01 to 0.98), and complex repetitive discharge (CRD) on the EMG (OR, 26.25; 95% CI, 2.67 to 258.52), versus those without. After adjustment for age, dysphagia and CRD remained significant, while ILD showed a trend for a difference but was not statistically significant. Multivariate analysis revealed that the CRD conferred an OR of 25.99 (95% CI, 1.27 to 531.86) for malignancy. When the frequency of malignancy was analyzed according to the number of risk factors, patients with three risk factors showed a significantly higher incidence of malignancy, versus those with fewer than two (p = 0.014).
Conclusions
We demonstrated for the first time that CRD on the EMG was an additional independent risk factor for malignancy in IIM. Further studies on a larger scale are needed to confirm the importance of CRD as a risk factor for malignancy in IIM.
INTRODUCTION
Idiopathic inflammatory myositis (IIMs), including dermatomyositis and polymyositis, is a group of chronic autoimmune conditions characterized by proximal muscle weakness and cutaneous lesions in dermatomyositis [1]. The risk of malignancy in patients with IIM, especially dermatomyositis, has been reported to be increased [2,3,4,5]. Risk factors associated with concomitant malignancies in IIM include older age at onset, cutaneous necrosis, increased erythrocyte sedimentation rate, and presence of cutaneous leukoclastic vasculitis [2,3,6,7,8]. The comorbidity of interstitial lung disease (ILD) and the absence of dysphagia are significantly preventative against malignancy [2,3]. However, previous studies have focused on demographic, clinical, and laboratory markers associated with malignancy in patients with IIM.
Uchino et al. [9] found that the incidence of rare infiltrative-type muscle pathology may be a predictive marker of dermatomyositis or malignancy in dermatomyositis. The results indicated that a subset of muscle inflammation might be specifically associated with malignancy. On electromyography (EMG), IIM is characterized by the presence of prominent muscle membrane irritability [10]. The degree of abnormal muscle membrane irritability is believed to reflect ongoing disease activity [10]. However, to date, no association between EMG findings and malignancies in IIM has been established.
Because malignancy is one of the most important complications affecting the prognosis of patients with IIM, an extensive evaluation for malignancy in patients with IIM has been recommended [3,4,6]. In this study, we investigated the EMG findings as well as demographic, clinical, and laboratory features that may predict the development of malignancy in patients with IIM.
METHODS
Patient enrollment
We conducted a retrospective study in patients with dermatomyositis and polymyositis admitted to Kyungpook National University Hospital between 1999 and 2013. The diagnoses of polymyositis and dermatomyositis were based on the criteria of Bohan and Peter [11]: (1) symmetric muscle weakness, (2) increased serum muscle enzymes, (3) myopathic changes on EMG, (4) typical histological findings on a muscle biopsy, and (5) characteristic dermatological manifestations. Two patients were diagnosed with amyopathic dermatomyositis (ADM) according to the criteria of Sontheimer [12]. Patients were divided into those with and without malignancies, and comparisons were made between the two groups in terms of their demographic factors, clinical findings, laboratory results, and EMG findings.
Patient demographics and clinical features
In this study, patients with hallmark cutaneous manifestations of dermatomyositis but for whom muscle testing did not reveal any abnormality were classified as having ADM, according to Sontheimer's criteria [12]. We included a total of 61 patients with dermatomyositis/polymyositis after excluding four patients with juvenile dermatomyositis, and two with ADM. We reviewed the medical records of these patients. The diagnosis of malignancy-associated IIM was retained if IIM occurred in the context of a recently diagnosed malignancy, or if a malignancy was diagnosed during the 2 years following the diagnosis of IIM (three patients were followed for less than 2 years after the diagnosis of IIM: two for 6 months and one for 9 months). Malignancy screening was performed in all patients, based on our institution's guidelines. All patients were screened for malignancy with laboratory tests including carcinoembryonic antigen, α-fetoprotein, carbohydrate antigen (CA 19-9), cancer antigen (CA-125), neuron-specific enolase, and β-human chorionic gonadotropin for females or prostate-specific antigen for males, radiographic imaging, including abdominal and chest computed tomographies (CTs), upper and lower endoscopic examinations, and positron emission tomography-CT if available. A muscle biopsy was performed in most cases. A nerve conduction study and needle EMGs were performed according to basic principles by one of two experienced electromyographers (JKS and HSS, Neurologist) with Medelec Synergy (VIASYS, London, UK). The classic triad of EMG findings of IIM (1) polyphasic, short duration motor unit potentials, (2) fibrillation, positive sharp waves, increased insertional activity, and (3) complex repetitive discharge (CRD) were evaluated [10,11].
Statistical analyses
Baseline characteristics were compared between groups using Student t test and the chi-square test, or Fisher exact test for categorical variables. An age-adjusted analysis was performed to compare malignancy-associated and nonmalignancy-associated IIM, using univariate logistic regression analysis. We calculated the odds ratios (ORs) and 95% confidence intervals (CIs) of the factors associated with malignancies in patients with IIM. The factors with a p value < 0.05 in the univariate analysis were subjected to a multivariate analysis. Multivariate logistic regression analysis was used to identify the relationship between the malignancy and predictor variables. The multivariate analysis used a step-wise backward regression model. The relative excess risk due to interaction (RERI) that was attributable to the interaction between the two variables [13] was calculated to estimate the excess risk. All statistical calculations were performed using the SPSS version 20 (IBM Co., Armonk, NY, USA). p values < 0.05 were considered to indicate statistical significance.
RESULTS
Demographic findings
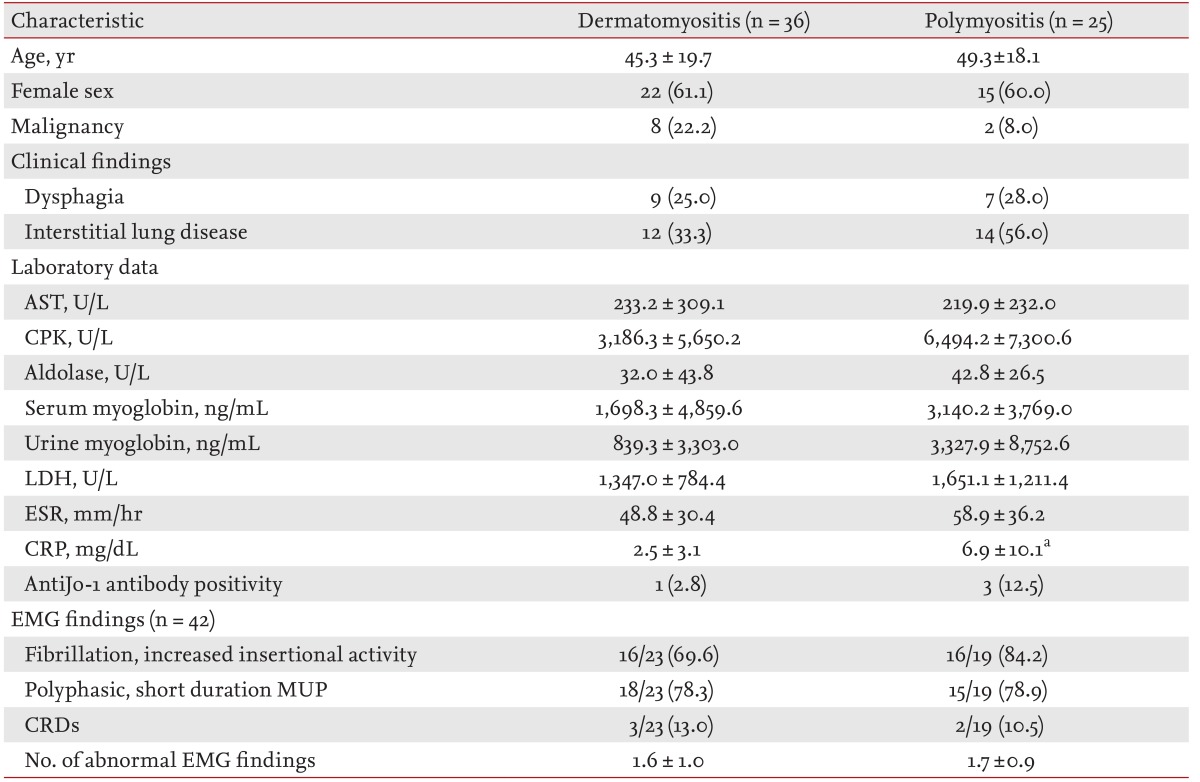
Each patient was followed from the time of the initial diagnosis at our institute to either the date of death or the date of the latest follow-up. We excluded patients with a preceding malignancy who had been in remission for more than 3 years at the time of IIM diagnosis. Among 61 the patients, dermatomyositis was the most common type (n = 30, 49%), followed by polymyositis (n = 25, 41%), juvenile dermatomyositis (n = 4, 7%), and ADM (n = 2, 3%). Demographic and clinical data on these patients are presented in Table 1. The mean age at diagnosis was 45.3 years for dermatomyositis (including ADM/juvenile dermatomyositis) and 49.3 years for polymyositis. Patients with IIM were predominantly women (60.7%). Distributions of age at diagnosis, gender, and frequency of ILD were not significantly different between the two groups.
Clinical characteristics of malignancy in IIM patients
Malignancies were present in 16.4% of all patients (10/61). These included eight of the 36 patients with dermatomyositis (including ADM and juvenile dermatomyositis) (22.2%) and two of the 25 with polymyositis (8.0%). No patient with ADM or juvenile dermatomyositis had a malignancy. There was no significant difference in the risk of malignancy between dermatomyositis and polymyositis patients. Gastric cancer was the most frequent malignancy observed (two patients), followed by bladder cancer, nasopharyngeal cancer, ovarian cancer, prostate cancer, thyroid cancer, lung cancer, gallbladder cancer, and invasive thymoma (one patient each). Adenocarcinoma was the predominant histological type (50%). A comparison of the clinical findings of the 10 patients with malignancies and 51 without is presented in Table 2.
Patients with malignancies showed a significantly higher incidence of dysphagia (OR, 21.50; 95% CI, 3.84 to 120.49; p < 0.001) and a lower incidence of ILD (OR, 0.12; 95% CI, 0.01 to 0.98; p = 0.034), compared with those without. After adjustment for age, dysphagia remained significant, while ILD showed a trend for a difference but was not statistically significant. Serum concentrations of muscle enzymes, such as creatine phosphokinase, aldolase, and myoglobin, and the frequency of autoantibodies, including antinuclear, and anti-Jo-1, -Ro, and -La antibodies, showed no significant difference between IIM patients with and without malignancies.
EMG was performed in 42 patients, including five with malignancy. The number of abnormal EMG findings was significantly higher in patients with malignancies (OR, 9.05; 95% CI, 1.08 to 75.57; p = 0.044). Patients with malignancies showed an increased incidence of CRD (OR, 26.25; 95% CI, 2.67 to 258.52; p = 0.008), versus those without, which remained significant after adjustment for age.
Factors associated with underlying malignancies
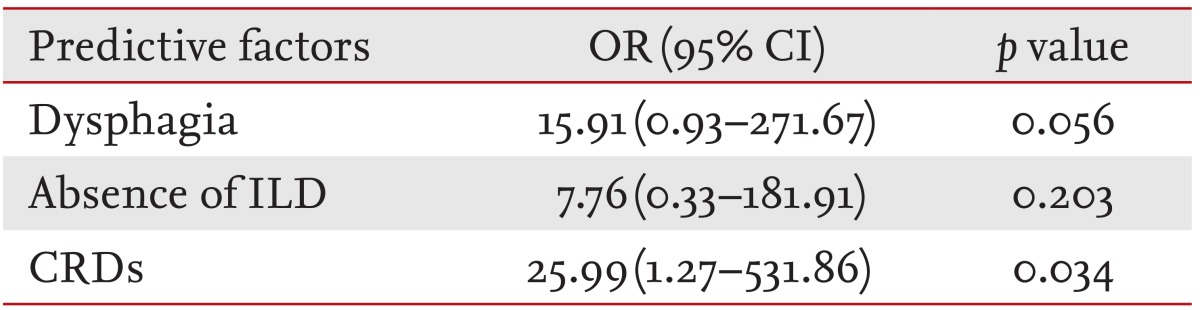
In the next step, interactions among the three predictive factors that were statistically significantly associated with a higher risk of malignancy in patients with IIM were investigated. We chose these significant factors for multivariate logistic regression to determine the independent predictive factors for malignancies (Table 3). The presence of CRD on the EMG conferred an OR of 25.99 (95% CI, 1.27 to 531.86; p = 0.034) for developing a malignancy. We found that CRD was another independent factor associated with malignancy in IIM, while dysphagia showed marginal statistical significance (p < 0.1). Furthermore, we found that an increase in the number of these risk factors was associated with a higher malignancy incidence. Patients with three risk factors showed a significantly higher incidence of malignancies, compared with those with zero (p = 0.007) or one (p = 0.014) (Fig. 1). RERI was calculated to determine the synergistic interaction between two variables among the three risk factors. There was no statistically significant excess risk attributable to an interaction between any two variables.

An increased number of risk factors is associated with a higher incidence of malignancy. Patients with three risk factors-dysphagia, absence of interstitial lung disease, and complex repetitive discharges on electromyography-showed a significantly higher incidence of malignancy, compared with those with no (p = 0.007) or one (p = 0.014).
DISCUSSION
Risk factors associated with malignancies in diagnostic tests, such as EMG, or pathology besides the clinical and laboratory features have rarely been defined in patients with IIM. In this study, we found that patients with malignancies had a higher incidence of CRDs on EMG as well as dysphagia and absence of ILD, versus those without malignancies. Moreover, multivariate logistic regression analysis revealed that the CRD stood out as another independent risk factor for malignancy in IIM. When the frequency of malignancy was analyzed according to the number of risk factors, patients with three factors had a significantly increased risk of malignancies, compared with those with fewer than two.
The standardized incidence ratios for malignancy in patients with dermatomyositis have been reported to be up to 14.2% [2,4,5]. The frequency of malignancies in patients with dermatomyositis and polymyositis has been reported to range from 15% to 35.7% and from 3.1% to 14.3%, respectively [2,3,4,6,7,14,15]. Our study showed that the frequencies of malignancies associated with dermatomyositis and polymyositis were 22% and 8%, respectively, within the range reported in previous studies, and there was no significant difference in the risk of malignancy between dermatomyositis and polymyositis patients.
Ovarian, lung, pancreatic, stomach, and colorectal cancers, and lymphomas have been reported as the most commonly associated malignancies in dermatomyositis [2,3,4]. In Asia, nasopharyngeal carcinoma is also reported frequently in IIM [2,7]. Among the various histological types of malignancy associated with IIM, adenocarcinoma is reported most frequently [4]. We also found that the most common histological type of the malignancies was adenocarcinoma (50%), although the origin of the malignancies varied.
Risk factors associated with concomitant malignancies in IIM include older age, dysphagia, absence of ILD, males, muscle enzyme levels, rapid onset of skin and/or muscular symptoms, skin necrosis, and periungal erythema, which have been reported in different combinations in various studies [2,3,6,7]. Of these factors, older age, dysphagia, and absence of ILD have been reported repeatedly as risk factors for malignancies. In this study, we also found that patients with malignancies had a higher frequency of dysphagia and absence of ILD, versus those without.
While the mechanism(s) behind the association between malignancy and dysphagia or absence of ILD have not been established, several hypotheses have been proposed. The association of dysphagia with malignancies might be explained by a mechanical component of gastric cancer or laryngeal muscle weakness due to cancer [2,7]. However, only two patients with gastric cancer and one with nasopharyngeal cancer were included in this study and that the significant difference between patients with and without malignancy was maintained after excluding these patients (85.7% vs. 15.7%, p < 0.001).
In the case of the negative association between ILD and malignancy, one possibility suggested in a previous study was that patients with ILD had a poor prognosis [16], indicating that they might not live long enough to develop malignancies [7]. However, the mean follow-up duration of patients with and without ILD was not significantly different in the present study, consistent with the findings of So et al. [2]. Another possibility is that the involvement of the muscular system in paraneoplastic syndrome may have a different mechanism, compared with that of IIM, which is associated with sparing of inflammatory cell infiltration in the lung.
Few studies have addressed the pathological characteristics of muscle in IIM with malignancy. In these studies, dermatomyositis patients with malignancies had less inflammatory cell infiltration and regeneration, versus those without [9,14]. Sampson et al. [17] reported that paraneoplastic necrotizing myopathy showed extensive necrosis but little or no lymphocytic infiltrate, possibly due to cytokine-mediated mechanisms of myocyte injury [18]. Consequently, humoral myocyte injury in malignancies associated with IIM, which shows the paucity of inflammatory cell infiltration, may induce chronic inflammation in the muscular system, thereby leading to muscular membrane irritability and a higher frequency of CRD on EMG.
CRD comprises trains of complex polyphasic potentials that repeat at a regular frequency (range, 5 to 100 Hz) and that characteristically begin and terminate abruptly [19]. The complex configuration of CRD is explained by the fact that it consists of action potentials generated by individual muscle fibers forming a closed circuit, activated through ephaptic transmission [20]. CRD is present in chronic diseases, such as inflammatory myositis, muscular dystrophies, and spinal muscular atrophy [20], while no reports on the association of CRD as a risk factor malignancy related with IIM, have to our knowledge been published. In the present study, we investigated additional risk factors and found that CRD was more frequently detected in patients with malignancies, versus those without. CRD on EMG together with dysphagia and the absence of ILD was analyzed by multivariate logistic regression, which identified CRD as another independent risk factor for malignancies.
We also evaluated the association between the frequency of malignancies and numbers of risk factors: CRD, dysphagia, and absence of ILD. Patients with all three factors had a significantly increased risk of malignancies, compared with those with fewer than two. However, there was no synergistic interaction between any two of the three risk factors. These data suggest that a higher number of risk factors may be a warning sign for hidden malignancies in IIM, thus suggesting the need for a thorough evaluation for malignancies. A limitation of our study was that it was a retrospective study at a single tertiary referral center, so some data were missing especially the results of EMG studies. Moreover, relatively few patients were enrolled.
In conclusion, we demonstrated for the first time that CRD on EMG was an independent risk factor for malignancies in IIM. Further studies on a larger scale are needed to confirm the importance of CRD as a risk factor for malignancies in IIM and to investigate the mechanism(s) underlying the association between CRD and the characteristic muscle pathology findings in IIM.
KEY MESSAGE
Patients with malignancies related to idiopathic inflammatory myopathy (IIM) had a higher incidence of complex repetitive discharge (CRD) on electromyography (p = 0.008) as well as dysphagia (p < 0.001) and the absence of interstitial lung disease (p = 0.034) versus those without malignancies.
Multivariate logistic regression analysis revealed that the CRD conferred an odds ratio of 25.99 (95% confidence interval, 1.27 to 531.86; p = 0.034) for malignancy.
A higher number of risk factors may be a warning sign for hidden malignancies in IIM, suggesting the need for a thorough evaluation for malignancies.
Acknowledgments
This research was supported by Kyungpook National University Research Fund, 2012.
Notes
No potential conflict of interest relevant to this article was reported.