The impact of high-flux dialysis on mortality rates in incident and prevalent hemodialysis patients
Article information
Abstract
Background/Aims
The effect of high-flux (HF) dialysis on mortality rates could vary with the duration of dialysis. We evaluated the effects of HF dialysis on mortality rates in incident and prevalent hemodialysis (HD) patients.
Methods
Incident and prevalent HD patients were selected from the Clinical Research Center registry for end-stage renal disease (ESRD), a Korean prospective observational cohort study. Incident HD patients were defined as newly diagnosed ESRD patients initiating HD. Prevalent HD patients were defined as patients who had been receiving HD for > 3 months. The primary outcome measure was all-cause mortality.
Results
This study included 1,165 incident and 1,641 prevalent HD patients. Following a median 24 months of follow-up, the mortality rates of the HF and low-flux (LF) groups did not significantly differ in the incident patients (hazard ratio [HR], 1.046; 95% confidence interval [CI], 0.592 to 1.847; p = 0.878). In the prevalent patients, HF dialysis was associated with decreased mortality compared with LF dialysis (HR, 0.606; 95% CI, 0.416 to 0.885; p = 0.009).
Conclusions
HF dialysis was associated with a decreased mortality rate in prevalent HD patients, but not in incident HD patients.
INTRODUCTION
The mortality rate of end-stage renal disease (ESRD) patients undergoing maintenance hemodialysis (HD) is higher compared with that of the general population [1]. More effective and well-tolerated HD treatment could improve the clinical outcomes of ESRD patients undergoing HD [2]. Dialysis duration and frequency, the nature of vascular access, and the quality of the dialysate influence the rates of short- and long-term complications in HD, which can affect the overall outcome. In particular, selection of the most effective dialyzer is an important determinant of HD treatment effectiveness. High-flux (HF) dialyzers, characterized by a higher degree of porosity compared with low-flux (LF) dialyzers, can clear a greater amount of medium-molecular-weight uremic toxins, such as β2-microglobulin, parathyroid hormone, advanced glycation products, and leptin [3,4,5,6,7].
Despite its superior ability to remove medium-molecular-weight toxins, the clinical benefit of HF dialysis remains controversial. Several observational studies indicated that HF dialysis confers a survival benefit [8,9,10,11,12]. However, two large, randomized clinical trials, the HEMO study and the European Membrane Permeability Outcome (MPO) study, reported no significant difference in the mortality rates associated with HF- and LF-dialysis [13,14,15,16].
This discrepancy could be due to differences in study design or population characteristics; we presently focus on the latter possibility. HF dialysis decreased the mortality rate in patients receiving > 3.7 years of dialysis prior to enrolment in the subgroup analysis of the HEMO study [9,10]. In contrast, there was no significant difference in the survival rates of HF versus LF dialysis patients receiving < 3.7 years of dialysis treatment prior to enrolment. This was also the case in the incident HD patients of the MPO study [13,14,15]. Furthermore, incident and prevalent HD patients differ in terms of the causes of, and risk factors for, mortality [17,18,19,20].
Therefore, we hypothesized that the impact on mortality rate of HF dialysis might differ between incident and prevalent HD patients, and might also vary in accordance with the duration of prior dialysis treatment in prevalent HD patients. This prospective cohort study, which included incident and prevalent HD patients, evaluated the impact of HF dialysis on mortality rates in incident and prevalent HD patients.
METHODS
Study population
Each patient enrolled to the study was on the Clinical Research Center registry for ESRD. An ongoing, observational prospective cohort design was employed, using patients with ESRD drawn from 31 centers in Korea. Enrolment commenced in April 2009, and included adult (> 18 years of age) incident and prevalent dialysis patients. Incident HD patients were defined as newly diagnosed ESRD patients initiating HD. Prevalent HD patients were defined as patients who had been receiving HD for > 3 months. Totals of 1,283 incident patients and 1,784 prevalent patients were included. Patients undergoing hemodiafiltration (n = 104) were excluded, as were patients for whom information regarding the type of dialysis membrane used was not available (n = 157). Totals of 1,165 incident and 1,641 prevalent patients were included in the final analysis.
Demographic and clinical data were collected at enrolment. Assessment of dialysis characteristics and measurements of health were conducted every 6 months, until the termination of the follow-up period. Dates and causes of death were immediately reported during the follow-up period. The study was approved by the Medical Ethics Committees of the participating hospitals. Informed consent was provided by all patients prior to their inclusion.
Clinical and dialysis parameters
Baseline demographic and clinical data, including age, sex, body mass index (BMI), type of dialysis membrane used, cause of ESRD, comorbidities, laboratory investigation results, and therapeutic characteristics, were recorded. Cardiovascular disease was defined as the presence of coronary heart disease, peripheral vascular disease, or cerebrovascular disease. Serum hemoglobin, total cholesterol, albumin, creatinine, and urea levels were determined from blood samples. To assess the effect of dialysis membrane type on mortality rates, patients were divided into HF and LF dialysis groups in accordance with the type of dialysis membrane used during their treatment. HF dialysis was defined as an ultrafiltration coefficient of ≥ 20 mL/mmHg per hour, and a sieving coefficient, for β2-microglobulin, of > 0.6. LF dialysis was defined as an ultrafiltration coefficient of < 20 mL/mmHg per hour, with a sieving coefficient for β2-microglobulin of 0.
A total of 26 types of LF dialyzer, and 31 types of HF dialyzer, were observed in this study. In incident HD patients, the most frequently used LF dialyzer was the Gambro polyflux 14L (36.9% of cases; Gambro, Lund, Sweden); the most frequently used used HF dialyzer was the Fresenius Medical Care F60S (26.9% of cases; Fresenius Medical Care, Bad Homburg, Germany). In prevalent HD patients, the most frequently used LF dialyzer was the Gambro polyflux 14L (30.7% of cases); the most frequently used HF dialyzer was the Fresenius Medical Care FX 60 (21.3% of cases). The types of dialysis membrane materials used were also determined. In incident HD patients, all of the dialysis membranes were synthetic in the HF dialyzer group; in the LF dialyzer group, 99.7% of the membranes were synthetic, and 0.3% contained substituted cellulose. In prevalent HD patients, all dialysis membranes were synthetic in the HF dialyzer group; in the LF dialyzer group, 98.4% of membranes were synthetic and 1.6% contained substituted cellulose. All dialysis sessions were conducted without reusing dialyzers, and all dialysate solutions were bicarbonate-based. The single-pool Kt/V (spKt/V: K, dialyzer clearance; t, time; V, volume of water contained within the body) was determined using two-point urea modeling on the basis of the intradialytic reduction in blood urea and intradialytic weight loss [21].
Outcomes
The primary clinical outcome measure was all-cause mortality. For each death, the clinical center principal investigator completed a form documenting cause of death according to the study's classification system.
Statistical analyses
Continuous variables with normal distributions are presented as means ± SD. Variables that are not normally distributed are presented as medians with ranges. Student t test, Mann-Whitney test, one-way analysis of variance and Kruskal-Wallis test were used, as appropriate, to assess differences in continuous variables. Categorical variables are presented as percentages. The Pearson's chi-square test or Fisher exact test was used to determine differences in categorical variables.
Absolute mortality rates were calculated per 100 person-years of follow-up. The primary outcome measure was mortality rate. All patients were followed until death (event), or until the study terminated, with censoring of data occurring either when patients underwent renal transplantation or were lost to follow-up following withdrawal of participation or transfer to a non-participating hospital. Survival curves for the HF and LF dialysis groups were estimated using the Kaplan-Meier method and the log-rank test. The Cox proportional hazard regression model was used to calculate the hazard ratio (HR), with 95% confidence intervals (CI), for all-cause mortality. For categorical variables, the assumption of proportional hazards over time was assessed by visual inspection of the log-minus-log survival plot. Analyses were adjusted for the following potential confounders: age, gender, BMI, diabetes mellitus, cardiovascular disease, causes of ESRD, duration of dialysis therapy, systolic blood pressure (BP), hemoglobin, serum albumin, serum total cholesterol, number of dialysis sessions per week, type of vascular access, and HD blood flow rates. To determine the interaction between the effect of HF dialysis on mortality and the duration of dialysis therapy in prevalent HD patients, we categorized the duration of prior dialysis therapy using quartiles, as follows (m): quartile 1, < 13.5; quartile 2, 13.5 to 33.0; quartile 3, 33.1 to 68.5; and quartile 4, > 68.5. Multivariate Cox regression analysis was performed for the different quartiles. A value of p < 0.05 was taken to indicate statistical significance. All analyses were performed using the SPSS version 11.5 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
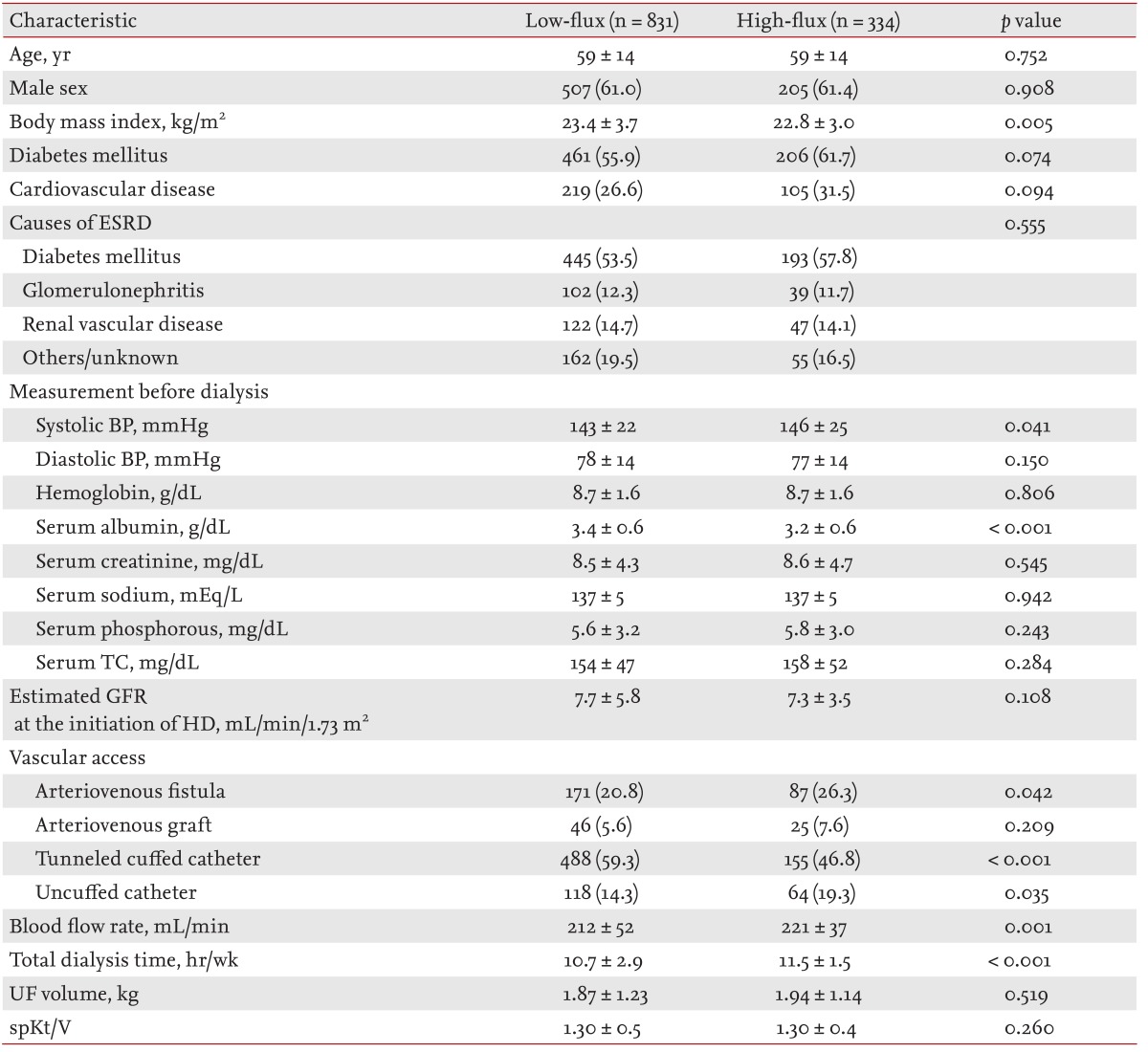
Totals of 1,165 incident and 1,641 prevalent patients were included in this study. Of the incident HD patients, 71.3% (831 of 1,165) were dialyzed using LF dialysis membranes; 28.7% (334 of 1,165) were dialyzed using HF dialysis membranes. The baseline characteristics of incident HD patients are displayed in Table 1. The HF group was characterized by lower BMI, higher systolic BP, and lower serum albumin levels compared with the LF group. There were no significant group differences in age, gender, prevalence of diabetes mellitus, prevalence of cardiovascular disease, causes of ESRD, diastolic BP, serum hemoglobin levels, serum total cholesterol levels or spKt/V. There was no significant group difference in the number of arteriovenous grafts used for vascular access. Significantly more tunneled cuffed catheters were used in the LF group compared with the HF group; but arteriovenous fistula and uncuffed catheter use was significantly more prevalent in the HF group (at the point of enrolment). The LF group was characterized by lower HD blood flow rates and fewer weekly dialysis sessions.
In 50.8% (834 of 1,641) of cases, prevalent HD patients were dialyzed using LF dialysis membranes, with 49.2% (807 of 1,641) of patients dialyzed using HF dialysis membranes (at the point of enrolment). The baseline characteristics of prevalent HD patients are shown in Table 2. The HF group was characterized by a lower prevalence of diabetes mellitus and longer durations of dialysis therapy prior to enrolment compared with the LF group. The duration of dialysis therapy in the LF group ranged between 3 and 360 months (median, 28; interquartile range, 11 to 57), compared with a range of 3 and 328 months (median, 39; interquartile range, 16 to 78) in the HF group. Diabetes mellitus, as a primary cause of ESRD, was more prevalent in the LF group. Systolic BP was higher, and serum total cholesterol levels were lower, in the HF group. There were no significant group differences in age, gender, BMI, prevalence of cardiovascular diseases, diastolic BP, serum hemoglobin levels, serum albumin levels or spKt/V; or in the frequency of use of tunneled cuffed catheters and uncuffed catheters (for vascular access). The frequency with which an arteriovenous fistula was used for vascular access was higher in the HF group, but arteriovenous grafts were used more frequently in the LF group (at the point of enrolment). The LF group was characterized by lower HD blood flow rates and fewer dialysis sessions per week.
Effect of membrane flux on all-cause mortality
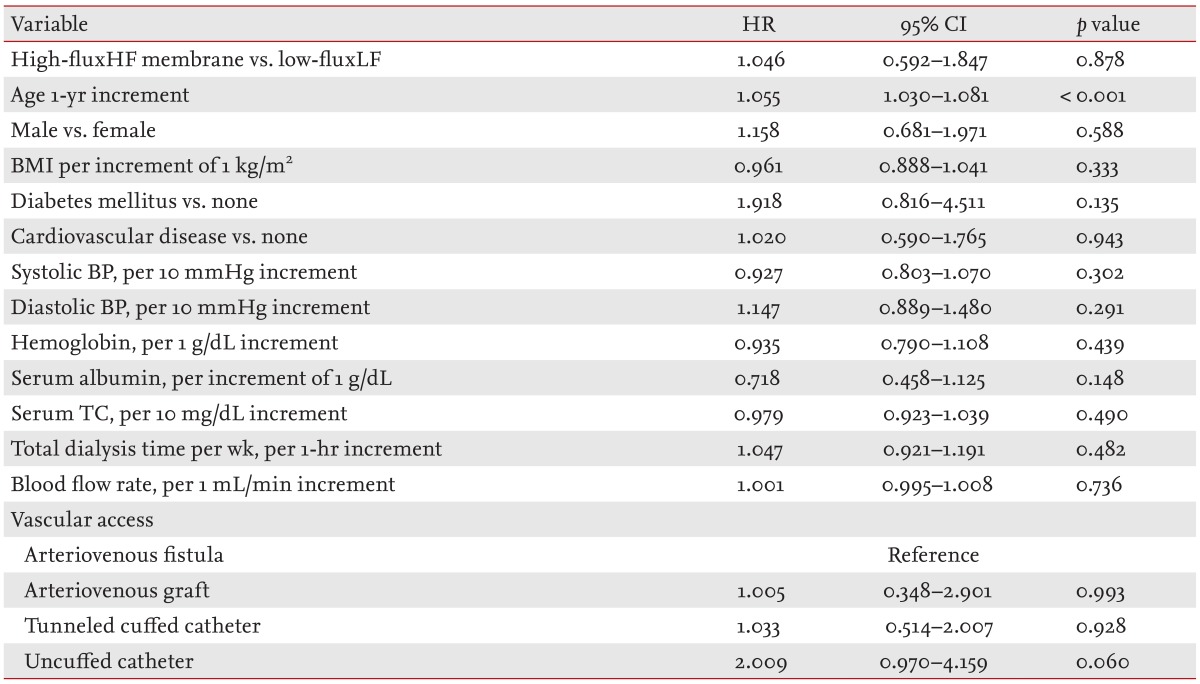
The median follow-up period was 24 months (interquartile range, 12 to 39). In total, 297 incident HD patients withdrew from the study for reasons other than death, comprising 43 patients who received kidney transplantation, 155 who were transferred to a nonparticipating hospital, 53 who withdrew their participation during treatment, and 46 who withdrew during the follow-up. Seventy-eight deaths occurred during the follow-up. The leading causes of death were cardiovascular events (30.6% of all deaths) and infectious diseases (26.4% of all deaths). The absolute mortality rate during the follow-up period was 4.4 deaths per 100 person-years. In univariate Cox regression analysis, HF dialysis was not associated with mortality (HR, 1.242; 95% CI, 0.761 to 2.029; p = 0.386). Fig. 1 depicts the Kaplan-Meier plot for all-cause mortality in incident HD patients according to HF and LF membrane use. There was no significant group difference (HF vs. LF) in survival rate (p = 0.384; log-rank test). Following adjustment for demographic variables, comorbidities, and laboratory data results, the adjusted HR for mortality in the HF group was 1.046 (95% CI, 0.592 to 1.847; p = 0.878), indicating that mortality rates for the incident HD patients did not differ significantly between the HF and LF groups (Table 3).

Kaplan-Meier survival curve for mortality rates associated with high-flux and low-flux membrane use in incident patients.
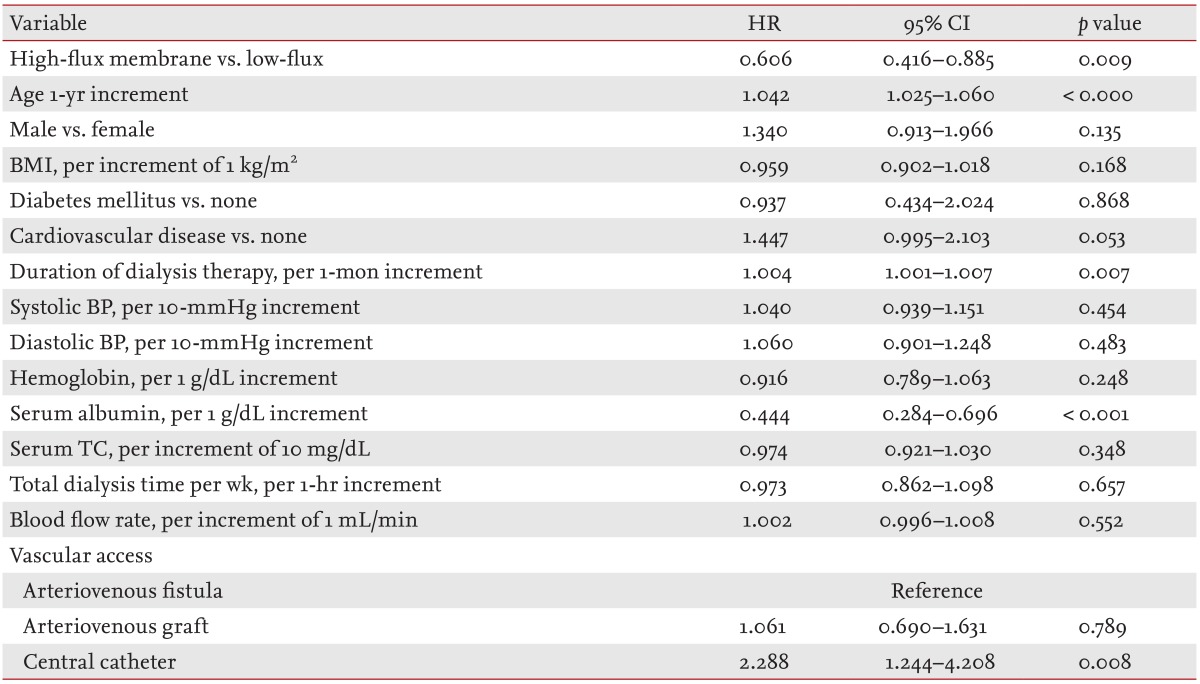
A total of 246 prevalent HD patients withdrew from the study for reasons other than death, comprising 71 patients who received kidney transplantations, 103 who transferred to a nonparticipating hospital, 38 who withdrew their participation during the treatment period, and 34 others who withdrew during the follow-up. One hundred and thirty-nine deaths occurred during the follow-up period. The leading causes of death were cardiovascular events (33.8% of all deaths) and infectious diseases (25.9% of all deaths). The absolute mortality rate during the follow-up period was 3.6 deaths per 100 person-years. In univariate Cox regression analysis, HF dialysis was associated with significantly reduced mortality rates (HR, 0.641; 95% CI, 0.454 to 0.906; p = 0.012). Fig. 2 depicts the Kaplan-Meier plot for all-cause mortality in prevalent HD patients according to HF or LF membrane use. Mortality was lower in the HF group compared with the LF group (p = 0.011; log-rank test). Even after adjusting for demographic variables, comorbid conditions and laboratory data, the adjusted HR for mortality was 0.606 (95% CI, 0.416 to 0.885; p = 0.009) in prevalent HD patients of the HF group: the HF group had a 39.4% lower risk of death compared with the LF group (Table 4).
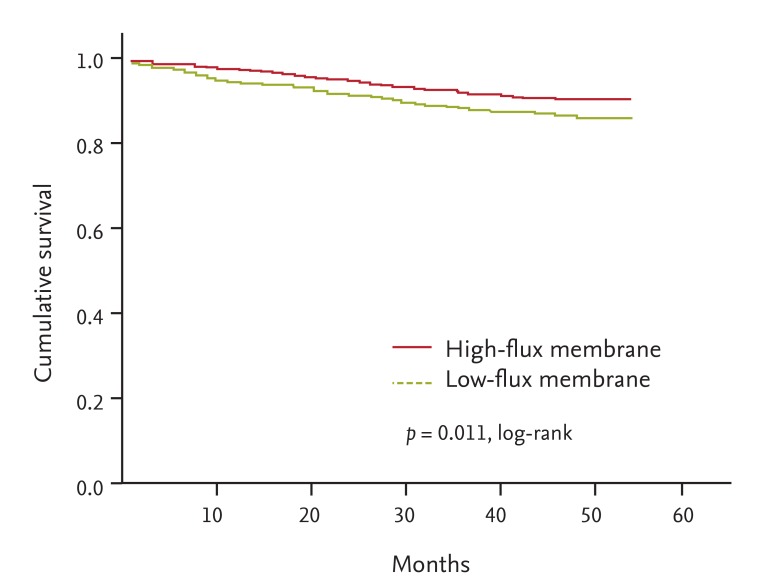
Kaplan-Meier survival curve for mortality rates associated with high-flux and low-flux membrane use in prevalent patients.
Interaction between membrane flux and the duration of prior dialysis in prevalent HD patients
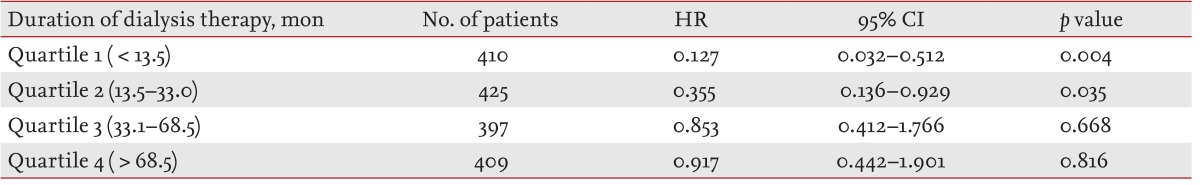
To determine the interaction between the effects of HF dialysis on mortality rate and the duration of dialysis therapy, in prevalent HD patients, we categorized the duration of prior dialysis therapy according to quartiles, as follows (m): quartile 1, < 13.5; quartile 2, 13.5 to 33.0; quartile 3, 33.1 to 68.5; and quartile 4, > 68.5. Multivariate Cox regression analysis indicated that HF dialysis was associated with a lower mortality rate in quartiles 1 (HR, 0.127; 95% CI, 0.032 to 0.512; p = 0.004) and 2 (HR, 0.355; 95% CI, 0.136 to 0.929; p = 0.035) compared with LF dialysis, with no significant group difference in mortality in quartiles 3 (HR, 0.853; 95% CI, 0.412 to 1.766; p = 0.668) or 4 (HR, 0.917; 95% CI, 0.442 to 1.901; p = 0.816) following adjustment for age, gender, use of an HF membrane, BMI, diabetes mellitus, cardiovascular disease, causes of ESRD, duration of dialysis therapy, systolic BP, diastolic BP, serum hemoglobin, albumin, or total cholesterol levels, total number of dialysis sessions per week, type of vascular access, and HD blood flow rate (Table 5).
DISCUSSION
In this prospective study, we demonstrated an association between HF dialysis and a decreased mortality rate in prevalent HD patients compared with LF dialysis. There was no significant difference between the HF and LF dialysis groups' mortality rates in incident HD patients. Our findings suggest that the impact of HF dialysis on mortality rates differs between incident and prevalent HD patients.
Our results accord with previous reports of a difference between incident and prevalent HD patients in the impact of HF dialysis on mortality rates. The survival benefit conferred by HF dialysis has also been described in several previous observational studies [8,9,10,11,12]. However, two large, randomized clinical trials, the MPO study (which included only incident HD patients) and the HEMO study (which included both incident and prevalent patients), revealed no significant difference in mortality rates between HF and LF dialysis groups [13,14,15,16]. The subgroup analysis of the HEMO study indicated that HF dialysis was associated with decreased all-cause mortality in patients receiving dialysis for > 3.7 years, prior to the commencement of the study, but not in the subgroup receiving dialysis for ≤ 3.7 years [14]. In our study, HF dialysis was associated with decreased all-cause mortality in a group of prevalent HD patients receiving dialysis for ≥ 3 months prior to study enrolment, but not in a group of incident HD patients.
The characteristics of the incident and prevalent patients in our study differed with respect to risk factors for mortality [17,18,19,20]. The mortality rate was highest in the first 3 months following the initiation of HD, and was also higher in incident compared with prevalent patients [17,18,19,20]. The most common risk factors for mortality in incident patients were the use of central venous catheters and inadequate predialysis nephrology care [17,18]. Because risk factors for mortality differ between incident and prevalent patients, this could confound any analysis of the effect of HF dialysis on the mortality rate in the whole cohort; therefore, we assessed the incident and prevalent patients separately.
Although there is no immediately obvious explanation for the differential impact of HF dialysis on mortality rates between incident and prevalent HD patients, several possible mechanisms can be proposed. First, central venous catheter access was employed more frequently in incident than in prevalent HD patients [22]. A central venous catheter was used in 71.5% of the incident HD patients (tunneled cuffed catheter, 55.7%; uncuffed catheter, 15.8%), compared with only 6.9% of the prevalent HD patients (tunneled cuffed catheter, 6.1%; uncuffed catheter, 0.8%). The low rate of venous catheter use in prevalent patients could have attenuated the survival benefit conferred by HF dialysis. Second, the mortality rate associated with withdrawal from dialysis was higher in the incident versus prevalent patients [17]. Bradbury et al. [17] reported that withdrawal accounted for 20% of deaths during the first 4 months following HD initiation, with 15% of deaths occurring in the subsequent 4 to 12 months. Higher death rates due to withdrawal in incident patients might overshadow the effects of HF dialysis on mortality. Third, previous exposure to LF or HF dialysis membranes could have affected the relationship between membrane flux type and mortality rate in the prevalent patients.
The interaction observed herein between the duration of prior dialysis, dialysis membrane flux type, and the mortality rate in prevalent HD patients is noteworthy. HF dialysis conferred a survival benefit in quartiles 1 and 2 of the duration of prior dialysis, but not quartiles 3 and 4. This suggests that, in prevalent HD patients, HF dialysis should be applied within 33 months following the initiation of dialysis, especially in prevalent patients who have received < 33 months of prior dialysis. The reason that HF dialysis conferred a survival benefit to prevalent patients who had received < 33 months of dialysis prior to entering the study is unclear. It is possible that longer durations of dialysis are associated with a greater accumulation of medium-molecular-weight toxins, such as β2-microglobulin [23]. The application of HF dialysis prior to the accumulation of significant amounts of such toxins could therefore be beneficial. However, our results are somewhat different to those of the HEMO study, in terms of the interaction between the duration of prior dialysis, the type of dialyzer membrane flux used and the mortality rate in prevalent HD patients. In the HEMO study, HF dialysis was associated with a lower risk of all-cause mortality, but only in quintiles in which patients had received > 6.09 years of prior dialysis [14]. Further studies are required to corroborate the relationships among HF dialysis, mortality rates and the duration of prior dialysis [24].
Our study had several limitations. First, we employed a prospective observational design. Accordingly, certain baseline characteristics differed between the HF and LF dialysis groups, especially in the context of the prevalent HD patients, thereby raising the possibility of selection bias. Randomized controlled trials comparing patients differing in terms of dialysis duration prior to enrolment are required to confirm the survival benefit conferred by HF dialysis according to the duration of prior dialysis. Second, the median follow-up period of 24 months was relatively short. Third, despite the multicenter nature of the study, the cohort consisted only of Korean patients. Thus, our results may not be generalizable to other, non-Asian ethnic groups receiving HD treatment.
In conclusion, HF dialysis was independently associated with decreased mortality compared with LF dialysis among prevalent HD patients, especially those who had received < 33 months of prior dialysis. However, HF dialysis conferred no significant survival benefit to incident HD patients. These findings suggest that HF dialysis differentially impacts upon mortality rates in incident versus prevalent HD patients, and further that the use of HF dialysis membranes should be recommended for prevalent patients within the 33 months following initiation of dialysis.
KEY MESSAGE
High-flux (HF) dialysis was associated with a decreased mortality rate in prevalent hemodialysis (HD) patients, but not in incident HD patients.
The use of HF dialysis membranes should be recommended for prevalent patients.
Acknowledgments
We thank the study coordinators Hye Young Lim, Nam Hee Kim, MiJoung Moon, Hwa Young Lee, Mi Joung Kwon, Su Yeon An, Su Joung Oh, and Hye Young Kwak for their contributions. This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI10C2020).
Notes
No potential conflict of interest relevant to this article was reported.