Survey of perinatal hepatitis B virus transmission after Korean National Prevention Program in a tertiary hospital
Article information
Abstract
Background/Aims
The Ministry of Health and Welfare and the Korea Centers for Disease Control and Prevention in South Korea have been organizing hepatitis B virus (HBV) vertical infection prevention projects since July 2002. In this single-institute study, the results of surveys conducted in target mothers who delivered babies in a tertiary hospital were investigated and analyzed.
Methods
Of the 9,281 mothers and their 9,824 neonates born between July 2002 and December 2012, 308 hepatitis B surface antigen (HBsAg)-positive mothers and their 319 neonates were selected for this study, and their records were analyzed retrospectively.
Results
A total of 308 mothers were HBsAg-positive, with an HBV prevalence of 3.32% (308/9,281). There were 319 neonates born to these HBsAg-positive mothers, and 252 were confirmed to as either HBsAg-positive or -negative. Four were confirmed as HBsAg-positive, with a 1.59% (4/252) HBV vertical infection rate. All the mothers of neonates who had an HBV vertical infection were hepatitis B e antigen (HBeAg)-positive. Among the HBsAg-positive neonates, three were HBeAg-positive and had an HBV DNA titer of 1.0 × 108 copies/mL.
Conclusions
The HBV prevalence of mothers was 3.32% (308/9,281), and their vertical infection rate was 1.59% (4/252). Thus, the South Korean HBV vertical infection prevention projects are effective, and, accordingly, HBV prevalence in South Korea is expected to decrease continuously.
INTRODUCTION
Hepatitis B is a major cause of acute/chronic hepatitis, cirrhosis, and liver cancer. Approximately 360 million people worldwide suffer from this infection, and 1 million die per year due to hepatitis-B-associated diseases [1]. According to the 2007 National Health and Nutrition Survey in South Korea, 3.7% of the population aged ≥ 10 years was infected with hepatitis B. Therefore, South Korea is classified as a medium-risk hepatitis B country [2,3].
Vertical infection is one of the major routes of chronic hepatitis B infection. In particular, in a country with a high hepatitis B e antigen (HBeAg)-positive rate, 40% to 50% of all chronic hepatitis B cases are caused by vertical infection [4,5]. Hepatitis B virus (HBV) infection becomes chronic more easily in the young than in the elderly; 80% to 90% of perinatally infected neonates experience chronic transition of hepatitis B compared with 5% in infected adults [6].
Since the introduction of immunoglobulin and hepatitis B vaccination in South Korea in 1980, the prevalence of hepatitis B in the general population and the vertical infection rate in neonates have been decreasing steadily [3]. In addition, the hepatitis B surface antigen (HBsAg)-positive rate in pregnant South Korean women has also decreased from 6.57% in the 1980s to 4.1% in 1990 and to 3.2% in 2006 [7,8,9].
When HBsAg-positive mothers did not undergo active-passive preventive measures using immunoglobulin and hepatitis B vaccines, their hepatitis B vertical infection rates increased abruptly. Approximately 70% to 90% of neonates from HBeAg-positive mothers who were not subjected to preventive measures contracted vertical infections, whereas only 10% to 20% of neonates from HBeAg-negative and HBsAg-positive mothers developed vertical infections, and about 90% of the infected neonates experienced chronic transitions [10,11]. Despite appropriate preventive measures, 5% to 10% of vertical infection cases still occurred [12,13]. The cause of the failure of the preventive measures has not yet been well explained, but it might be associated with the mother's serum HBV DNA titer and intrauterine infection. The higher the mother's serum HBV DNA titer level, the greater the increase in vertical infection risk [12,13,14,15,16,17].
To reduce hepatitis B prevalence and vertical infection in Korea, the Ministry of Health and Welfare and Korea Centers for Disease Control and Prevention (KCDCP) have been organizing hepatitis B vertical infection prevention projects since July 2002. In this study, the preliminary results of such projects involving mothers who delivered babies at Gachon University Gil Medical Center between July 1, 2002 and December 31, 2012 were investigated and analyzed.
METHODS
Patient enrollment
A total of 9,281 South Korean mothers and their 9,824 neonates who were born at Gachon University Gil Medical Center between July 1, 2002 and December 31, 2012 were selected for this study. This study was conducted during the implementation of the hepatitis B vertical infection prevention project by the South Korean government.
Methods and materials
The medical records of 9,281 Korean mothers and their 9,824 neonates who were born at Gachon University Gil Medical Center between July 1, 2002 and December 31, 2012 were reviewed. Among them, 308 mothers were confirmed to be HBsAg-positive and to have delivered 319 neonates. The time when these mothers were diagnosed with hepatitis B infection, their use of antiviral agents, and their history of hepatitis, cirrohosis, and liver cancer were investigated. Additionally, the results of biochemical tests, including aspartate aminotransferase/alanine aminotransferase (AST/ALT), HBeAg, hepatitis B e antibody (HBeAb), HBV DNA, and α-fetoprotein (AFP) were confirmed. Moreover, we tested whether their neonates were HBsAg-positive or not.
The gestation period, method of delivery, Apgar score, and schedule of hepatitis B vaccinations of the neonates were investigated. When a neonate was HBsAg-positive, the results of biochemical tests, including HBeAg, HBeAb, HBV DNA, AFP, and AST/ALT, were investigated. Additionally, information on abdominal ultrasound of the mothers and their neonates was collected. Any further information needed for this study was collected through telephone interviews. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee of Gachon University Gil Medical Center.
RESULTS
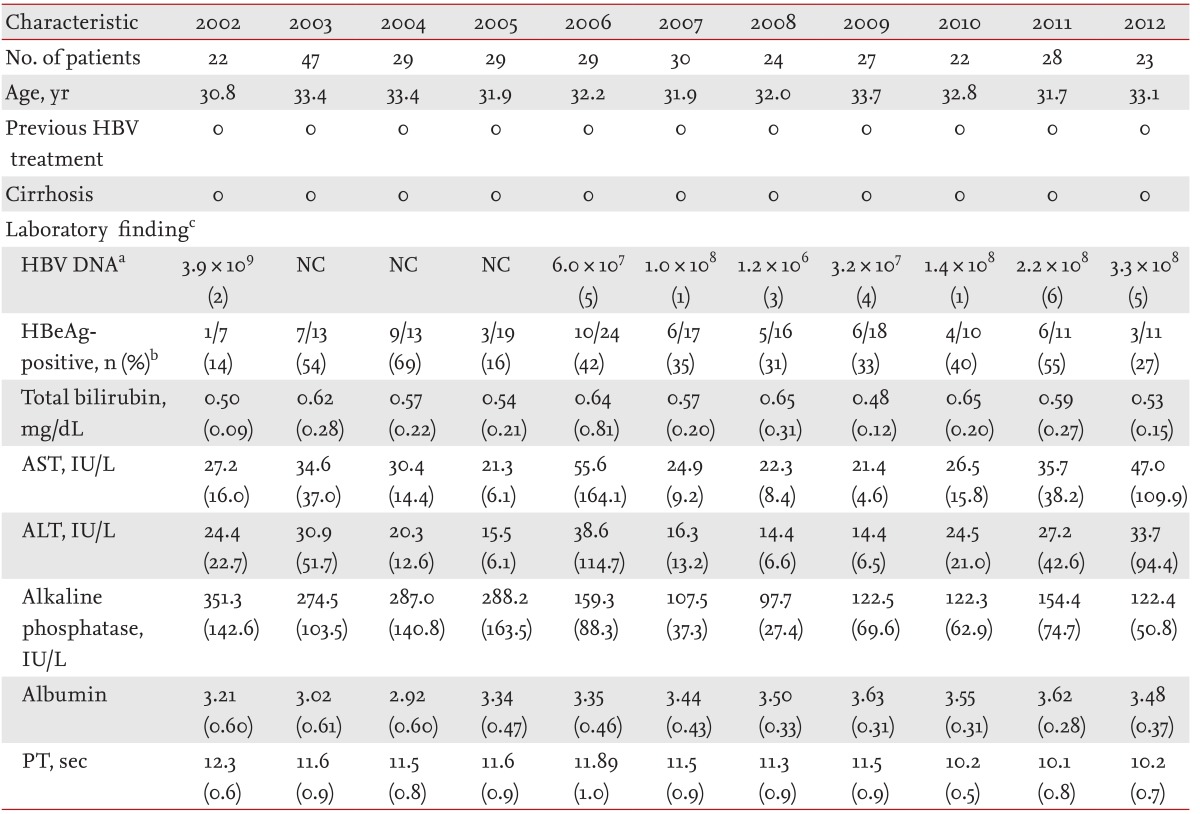
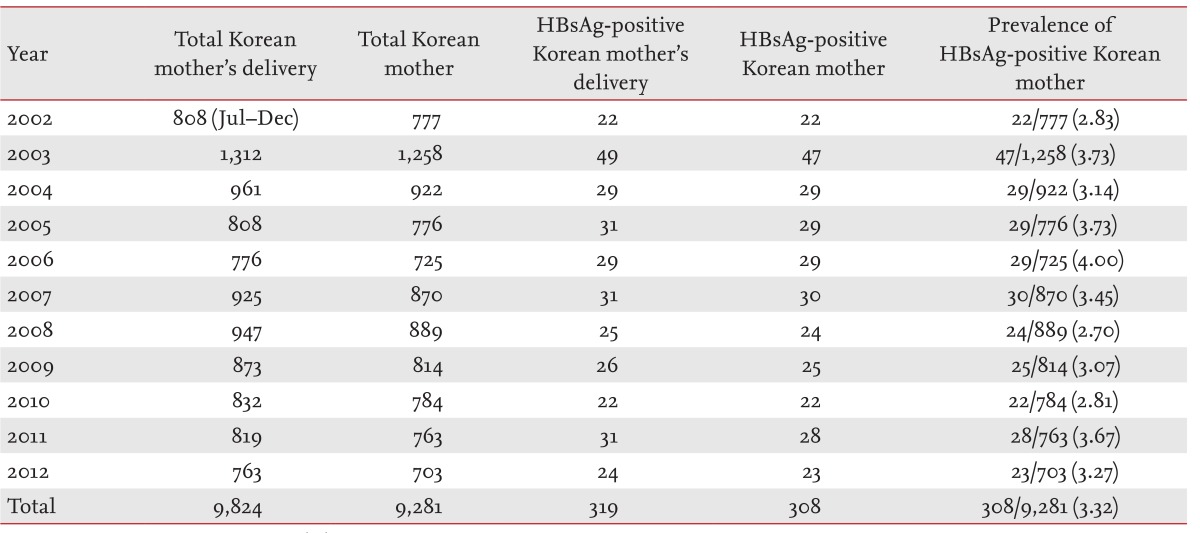
Between July 1, 2002 and December 31, 2012, 9,281 South Korean mothers gave birth to 9,824 neonates at Gachon University Gil Medical Center. Among the mothers, 308 were HBsAg-positive and delivered 319 neonates. During the same period, these mothers' hepatitis B prevalence was 3.32% (308/9,281). Their annual hepatitis B prevalence was 2.83% (22/777) in 2002, 3.73% (47/1,258) in 2003, 3.14% (29/922) in 2004, 3.73% (29/776) in 2005, 4.00% (29/725) in 2006, 3.45% (30/870) in 2007, 2.70% (24/889) in 2008, 3.07% (25/814) in 2009, 2.81% (22/784) in 2010, 3.67% (28/763) in 2011, and 3.27% (23/703) in 2012 (Table 1).
Prevalence of hepatitis B surface antigen-positive mothers at Gachon University Gil Medical Center from July 2002 to December 2012
Of the 319 neonates from HBsAg-positive mothers, 11 stillborn babies were excluded, and 252 were tested for confirmation of their HBsAg status. Of these 252 subjects, four were confirmed for HBsAg positivity, indicating a vertical infection rate of 4/252 (1.59%) (Table 2). Of these four neonates, two were born in 2002, one, in 2003, and one in 2004. No case of vertical infection was reported during 2005 to 2012. Immunoglobulin was injected in all neonates immediately after birth, and all were administered hepatitis B vaccinations 12 hours, 1 month, and 6 months after birth.
The HBeAg-positive rate in HBsAg-positive mothers was 37.74% (60/159), and the mean serum HBV DNA titer was 4.3 × 108 copies/mL. However, only 159 of the 308 HBsAg-positive mothers underwent the HBeAg test, and only 27 of these the serum HBV DNA titer test. None of the mothers received treatment with antiviral agents during pregnancy, and none suffered from liver cirrhosis at the time of delivery (Table 3).
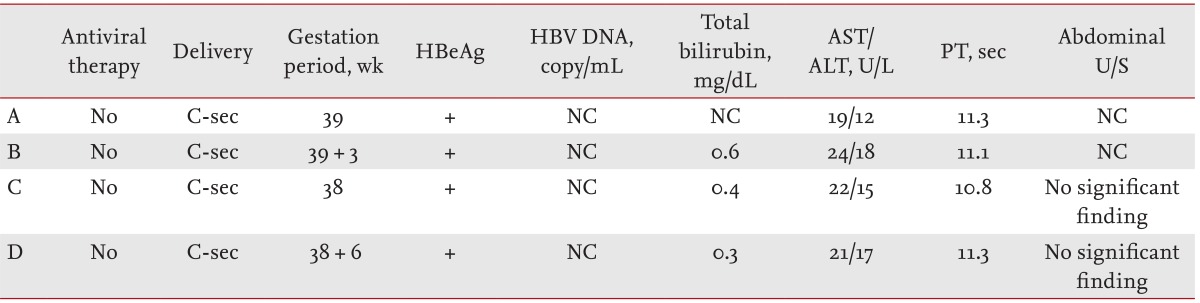
Despite the preventive measures taken with the immunoglobulin and hepatitis B vaccines, four neonates developed a vertical infection. Their mothers were HBeAg-positive, but their HBV DNA titers were not investigated. All subject mothers showed normal levels on biochemical tests, and no specific radiological findings were detected (Table 4).
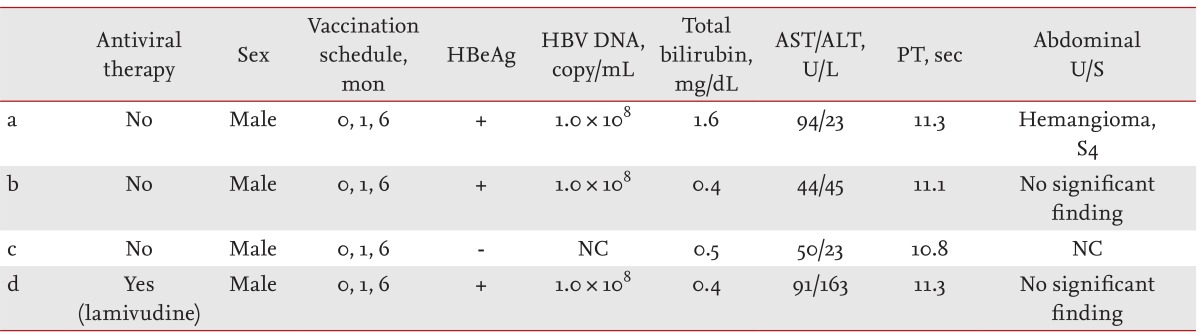
All four of the neonates who were infected with hepatitis B were male, and three were HBeAg-positive and showed 1.0 × 108 copies/mL of HBV DNA. The remaining neonate was HBeAg-negative, but the serum HBV DNA level was not measured. Immunoglobulin was injected immediately after birth in the hepatitis B-infected neonates, and the hepatitis B vaccination was administered as scheduled at baseline and at 1 month and 6 months after birth. In all infected neonates, moderate increases in AST and ALT levels were observed, and hemangioma was confirmed in one neonate by abdominal ultrasound (Table 5). The HBsAg-positive babies are currently being monitored in the Pediatric Department of Gachon University Gil Medical Center. Among them, one was administered an antiviral agent (lamivudine 100 mg per day) between March 2006 and May 2009. In September 2010, this neonate tested negative for both HBeAg and HBV DNA and positive for HBeAb.
DISCUSSION
Before hepatitis B vaccination was introduced in South Korea, the hepatitis B prevalence had reached 8% to 10% in the general population, and the country was classified as high-risk for this disease [3,7]. After hepatitis B vaccination was introduced in 1983, it was registered in the Infant Vaccination Guidelines in 1991 as a basic vaccination for infants; in 1995, it was included in the universal vaccination package for all neonates. Consequently, a significant decrease in the HBsAg-positivity rate has been observed in infants born after 1995 [18]. In July 2002, the Ministry of Health and Welfare of South Korea and the KCDCP initiated hepatitis B vertical infection prevention projects to lower the chronic hepatitis B prevalence in neonates down to 0.1% in 10 years. In addition, the HBsAg-positive rate of the general population is expected to decrease to 1% or lower, and the hepatitis B-induced liver cancer prevalence is expected to decrease to one-tenth in 20 years through these projects. More than 10 years have passed since the launch of the aforementioned projects, but studies and evaluations of their results to date have been insufficient. One such study was reported in 2005 [19], but it concerned the hepatitis B prevalence among mothers in 2001 and 2002, when the aforementioned projects had not yet been conducted. In addition, the other study was concerned with investigating the method of administering immunoglobulin and hepatitis B vaccinations to neonates and with breast feeding in HBsAg-positive mothers. No study on the hepatitis B vertical infection rate has been reported. In the present study, the results of the hepatitis B vertical infection prevention projects in mothers who delivered babies in Gachon University Gil Medical Center between July 1, 2002 and December 31, 2012 were investigated and evaluated.
According to studies on the HBsAg-positivity rate in pregnant South Korean women, the rates were 6.57% in the 1980s, 4.1% in 1990, 3.8% in 1991, 3.6% in 1992, 3.5% in 1993, 3.4% in 1994, 3.4% in 1995, 3.2% in 2001, and 3.2% in 2006 [7,8,9]. In this study, the rate of HBsAg-positive mothers who gave birth in Gachon University Gil Medical Center between July 2002 and December 2012 was confirmed as 3.32%, which is similar to that reported in the previous studies. Based on our results and those of previous studies, the rate of HBsAg-positive pregnant women decreased abruptly in the 1980s, but since 1990, it has remained at approximately 3% or increased slightly. According to the Korea National Nutrition Survey in 2011, the rate of HBsAg-positive females in their 10s was 0.3%; in their 20s, 1.4%; and in their 30s, 3.5% [20]. The low rates of HBsAg-positive females in their 10s and 20s are expected to further decrease in the future when these females marry and give birth. Accordingly, the incidence of hepatitis B vertical infection of future babies is also expected to decrease significantly. However, the decrease in rate of HBsAg positivity in mothers is slowing more than expected as the age at marriage and first birth are rising in South Korea.
In this study, four of the 252 neonates who tested positive for HBsAg were confirmed to be such despite appropriate immunoglobulin and hepatitis B vaccinations; thus, the vertical infection rate was 1.59% (4/252). This rate is lower than those reported in two other studies (7.4% in Korea in 2008 [21] and 2.1% in Canada in 2007 [22]) and similar to that of another study (1.54% in China in 2013 [16]).
All mothers who had vertically infected neonates were HBeAg-positive, but no test on their HBV DNA titer was conducted. However, since they were HBeAg-positive, we could speculate that all of them had a high HBV DNA titer that increased the possibility of vertical transmission. Of the 308 HBsAg-positive mothers, 27 underwent a serum HBV DNA titer test, of whom one showed an HBV DNA titer of 1.02 × 108 copies/mL, even though she was negative for HBeAg. She was considered HBeAg-negative with a precore- or core-promoter mutant. According to a KCDCP survey in 2004, hepatitis B vertical infection developed in 30.8% of HBeAg-negative mothers with an HBV DNA titer of 5 × 107 copies/mL or higher, despite having received the appropriate vaccinations [17,18]. When the HBV DNA titer was 107 IU/mL or higher, the hepatitis B vertical infection rate was 6.01%, and when the titer was lower than 107 IU/mL, the rate was 0.19% [16]. Therefore, HBeAg should be tested in HBsAg-positive pregnant women, and an additional HBV DNA titer test may be needed even in HBeAg-negative cases.
Since a high HBV DNA titer is considered to be the major reason for vaccination failure, administration of antiviral agents during late pregnancy has been suggested in many studies. According to a meta-analysis of 15 studies, administering lamivudine during late pregnancy significantly lowered the HBsAg-positivity rate of 6- to 12-month-old infants who were born from HBsAg-positive mothers, compared with cases in which only immunoglobulin and hepatitis B vaccinations were administered (relative risk [RR], 0.33; 95% confidence interval [CI], 0.21 to 0.50; p < 0.001). The side effects observed in the lamivudine-administered group were just as severe as in the control group (p = 0.97), and only one study has reported side effects [23]. According to a meta-analysis of telbivudine, its use in late pregnancy significantly lowered the HBsAg-positivity rate of 6- to 12-month-old infants who were born to HBsAg-positive mothers, compared with cases in which only immunoglobulin and hepatitis B vaccinations were used (RR, 0.11; 95% CI, 0.04 to 0.31; p < 0.001). No side effects were reported in the mothers and neonates administered telbivudine [24]. Based on these results, the use of antiviral agents in HBsAg-positive pregnant women during late pregnancy may reduce their serum HBV DNA titer and vertical infection rate.
Yogeswaran and Fung [25] suggested measuring the HBV DNA titer of all HBsAg-positive mothers during the second trimester of pregnancy. With HBV DNA titers greater than or equal to 7 log10 IU/mL, they recommended the use of lamivudine, tenofovir, and/or telbivudine during late pregnancy. Nevertheless, there is no consensus among experts on the use of antiviral agents in HBsAg-positive mothers to prevent vertical infection, but careful use may be necessary considering the treatment period, the timing of the terminating treatment, the risk of resistance, and patient preference [26]. Four infants were HBsAg-positive and three infants HBeAg-positive in our study. Although the HBV DNA level was not measured in all HBsAg-positive infants, they may have had high viral loads. In one recent Chinese study, 12 (4.82%) infants whose mothers were HBsAg-positive were infected, and all of the infants were HBeAg-positive with high viral loads despite vaccination [27]. They did not complain of any symptoms, but an HBsAg test should be conducted for these infant cases for public health control.
In this study, which was conducted after the launch of the hepatitis B vertical infection prevention projects by the South Korean government, the vertical infection rate was confirmed as 1.59% (4/252), so the projects are considered to be very effective. In particular, considering that the hepatitis B prevalence among South Korean females in their 10s and 20s is now very low (0.3% to 1.4%), future hepatitis B prevalence is expected to decrease consistently.
The limitations of this study are as follows: it was a single-institute retrospective study; it consisted of 56 neonate subjects whose HBsAg status could not be followed up; and HBeAg and HBV DNA titer tests were not conducted in all HBsAg-positive mothers. However, this study is notable in that it reviewed the trend of prevention projects conducted since July 2002 and evaluated the utility of such projects. In the future, a multi-institute prospective study may be necessary to identify a novel preventive measure to lower hepatitis B prevalence.
KEY MESSAGE
Hepatitis B virus (HBV) vertical transmission rate was 3.32% in hepatitis B surface antigen-positive mothers with HBV vertical infection prevention projects.
HBV vertical infection prevention projects in South Korea are effective and, HBV prevalence in South Korea is expected to decrease continuously by this prevention projects.
Notes
No potential conflict of interest relevant to this article was reported.