Expression of procaspase 3 and activated caspase 3 and its relevance in hormone-responsive gallbladder carcinoma chemotherapy
Article information
Abstract
Background/Aims
The higher incidence of gallbladder cancer (GBC) in females has been accredited to the involvement of hormones. The clinical implications of sex hormone receptors in GBC are well established. Cysteine proteases (such as caspase-3-9, etc.) are known to play a central role in the apoptotic pathway. Of these, the downstream enzyme caspase-3 is often activated in the apoptotic pathway. The aim of this work was to examine the status of apoptosis (which directly correlated with the level of active caspase-3) in hormone-responsive GBC.
Methods
We used 10 androgen receptor (AR)-positive, 14 estrogen receptor (ER)-positive, 12 HER/neu-positive, eight triple positive, and 10 triple negative malignant GBC human tissue samples. We isolated the total cellular protein from tumor tissues and carried out Western blotting using antipro-caspase-3 and anti-activated caspase-3 antibodies.
Results
ER and HER/neu-positive GBC exhibited high caspase-3 activity and low procaspase-3 activity, whereas AR-positive GBC showed no significant level of apoptosis. We also evaluated the apoptosis status of triple positive GBC and triple negative GBC, and found significant apoptosis in triple positive GBC.
Conclusions
The results indicate that ER and HER/neu-positive GBCs had active apoptosis, whereas AR-positive GBC was highly resistant to apoptosis.
INTRODUCTION
Gallbladder carcinoma (GBC) is a gastrointestinal and often fatal malignancy, ranked fifth overall and the third most common biliary tract malignancy [1,2]. Correct diagnosis at a time which would enable a cure for GBC remains problematic due to its asymptomatic nature during the initial stages [3,4]. In general, it is one of the most aggressive biliary cancers with a very short survival duration [5]. The higher incidence of GBC in females has been attributed in part to hormonal factors, including estrogen and progesterone receptor expression and their effects on GBC prognosis [6,7]. Many other reports have also referred to the effects of hormone receptor status in GBC [8-14]. More recently, a significant correlation between hormone receptor polymorphism and gallbladder (GB) carcinogenesis has been reported [15]. Androgen receptor (AR) CAG repeats have been directly correlated with gallstone formation and GB carcinogenesis [16]. These studies have shown that hormone receptors are important for GB carcinogenesis, with whole GB resection being the preferred curative treatment [17]. Adjuvant chemotherapy and molecular-targeted therapy are potential therapeutic options for advanced-stage cancer. In GBC, due to its typically late diagnosis and presentation, chemotherapy is administered before surgical removal of the GB tumor. It is therefore vital to develop new drugs for GBC, with a view to combating the cancer at the molecular level.
There are 14 caspases in mammals [18,19]. The initiator caspases (e.g., caspases 8, 9, and 10) are activated by apoptotic stimulation, which activates further effector caspases [20]. Additionally, many studies have shown that active caspase-3 is needed to induce apoptosis in response to chemotherapeutic treatments using regimens such as taxanes, 5-fluorouracil, and doxorubicin [21-24]. Caspase-3 is synthesized as a 32-kDa proenzyme, which is cleaved into 12- and 17-kDa subunits. Two 12-kDa and two 17-kDa subunits are reassociated to form the functionally active caspase-3 enzyme [25]. After activation, the effector caspases, which include caspase-3, -6, and -7, initiate the cleavage of many key cellular proteins, including poly (ADP-ribose) polymerase, inhibitors of caspase-activated DNase, gelsolin, 4-GDI, α- and β-fodrin, and epidermal growth factor receptors [19,26]. These cellular proteins cause blebbing of the membrane, condensation of chromatin and DNA fragmentation. Based on the central role played by caspase-3 in apoptosis, we assessed the expression levels of procaspase-3 and activated caspase-3 in advanced GB tumor tissue samples to determine the apoptotic status of estrogen receptor (ER)-, AR-, and HER/neu-positive GBC tissues.
METHODS
Sample collection
GB tumor tissue was collected from the surgical oncology operation theater at the Institute of Medical Sciences, Banaras Hindu University, Varanasi, India by H.S.S. and M.T. Tumor samples were frozen in liquid nitrogen and stored at -80℃. Parts of the tumors were sent to the Department of Pathology at the Institute of Medical Sciences, Banaras Hindu University for histopathology and hormone receptor profiling. Well-characterized and histopathologically proven malignant tumors were selected. A total of 10 AR-positive, 14 ER-positive, 12 HER/neu-positive, eight triple positive, and 10 triple negative malignant tissue samples were selected for this study. None of the patients had received preoperative radiotherapy or adjuvant chemotherapy. This study was approved by the Human Ethics Committee of the Institute of Medical Sciences, Banaras Hindu University, Varanasi, India.
Protein isolation and Western blotting
Protein isolation and quantization were carried out according to methods described previously [27]. Equal amounts of GB tissue cellular proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. The proteins were electrotransferred to a nitrocellulose membrane. Antisera against procaspase-3 (ab13586, Abcam, Cambridge, MA, USA), active caspase-3 (Cell Signaling, Boston, MA, USA), and β-actin (Sigma, St. Louis, MO, USA) were used, with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) depending on the primary antibody. The specific bands were visualized by enhanced chemiluminescence (Abcam). Autoradiograms were quantified by densitometry using Alpha Innotech software (Alpha Innotech, San Leandro, CA, USA). The same membrane was reprobed with a β-actin-specific antibody as a loading control. Relative protein levels were calculated compared to the β-actin standard.
Statistical analysis
Student t test was used to determine significance levels (SPSS version 11, SPSS Inc., Chicago, IL, USA) in paired data. For multiple comparison, a two-way analysis of variance was used. Differences with value of p < 0.05 were considered significant.
RESULTS
Caspase-3 expression in triple positive and negative hormone receptors of GBC
Procaspase-3 and activated caspase-3 expression was evaluated in AR-, ER-, and HER-positive and negative GBC samples. In triple negative GBC, the mean levels of procaspase-3 and activated caspase-3 expression were similar (p < 0.834). Activated caspase-3 expression was significantly higher than that of procaspase-3 in triple positive GBC (p < 0.01) (Fig. 1). The ratio of procaspase-3 to activated caspase-3 was 0.98 in triple negative, and 0.803 in triple positive samples. These data indicate that the apoptotic rate of triple positive GBC is ~17.7% higher than that in triple negative GBC.
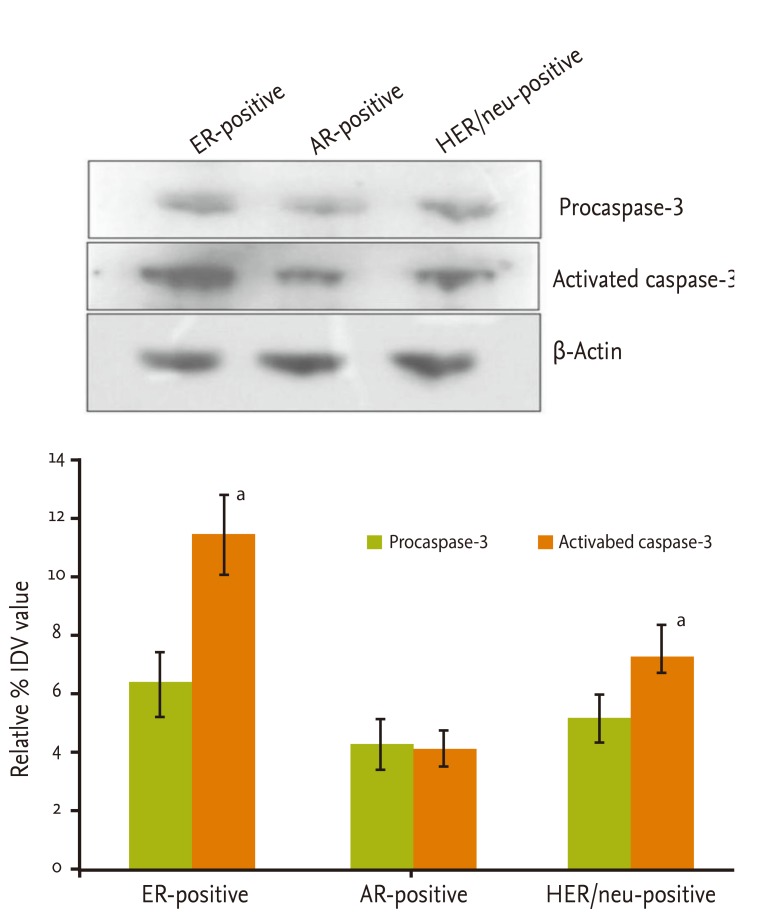
Caspase-3 expression in AR-, ER-, and HER/neu-positive GBC
In triple negative GBC, no significant differences were observed between procaspase-3 and activated caspase-3 levels. Further, expression of procaspase-3 and activated caspase-3 proteins was assessed in only AR-, ER-, and HER/neu-positive GBC. The maximum expression level of caspase-3 was observed in ER-positive GBC (2.1 times higher than AR-positive, 1.4 times higher than HER/neu-positive), followed by HER/neu- (1.4 times higher than AR-positive) and AR-positive GBC. The expression of activated caspase-3 was significantly higher than that of procaspase-3 in ER-positive cases (p < 0.01). There was no significant difference between procaspase-3 and active caspase-3 expression in AR-positive (p < 0.845) cases. HER/neu-positive cases were similar to ER-positive cases. Activated caspase-3 expression was significantly higher than that of procaspase-3 (p < 0.0346) (Fig. 2). Overall, ER- and HER/neu-positive cases were similar in their molecular nature, whereas AR-positive cases exhibited a difference in the expression of the apoptosis-functional protein caspase-3.
DISCUSSION
Chemoresistance is a principal cause of treatment failure in many cancers. To date, our understanding or resistance to cancer drugs remains limited. In order to identify and study hormone receptor expression and apoptosis rates, we have assayed procaspase-3 and active caspase-3 proteins in GB carcinoma. We show here that a triple negative hormone receptor GBC demonstrates less active apoptosis, whereas a triple positive hormone receptor expressing GBC demonstrates more active apoptosis. This indicates that expressing GBC hormone receptors may be more sensitive to hormone-dependent chemotherapeutic agents. As hormone receptors play a role in cancer initiation, it is important to study the status of apoptosis in hormones involved in GBC. Endocrine therapy using tamoxifen, a selective ER modulator, and aromatase inhibitors, which ablate peripheral estrogen synthesis, have been shown to substantially improve disease-free survival [28]. We first assessed procaspase-3 and active caspase-3 expression in triple negative and triple positive GBC so as to identify a correlation between hormone receptor expression and the rate of apoptosis. This information is clinically important because caspase-3 activation is necessary for initiation of apoptosis and regulation of processes such as membrane blebbing and internucleosomal DNA fragmentation. Caspase-3 expression in the MCF-7 human breast cancer cell line (caspase-3 deficient) revives the apoptotic response [28-30]. Caspase-3 is also involved in breast cancer apoptosis when cells are exposed to anthracyclines [23,31,32] and cisplatin [33-36]. Apoptosis failure is a crucial step in the initiation and progression of cancer. High apoptotic rates have been reported in invasive cancers compare to the paired normal rates [37-40]. We found no significant difference between procaspase-3 and active caspase-3 expression in triple negative GBC. However, we found a significantly higher active caspase-3 concentration in triple positive GBC, which exhibits high apoptotic activity. Our results show that triple negative GBC is more resistant to apoptosis than triple positive GBC. This information can be used clinically to select chemotherapeutic drugs in a hormone-dependent or independent manner. For therapeutic chemotherapy, triple positive GBC is more sensitive to apoptosis-inducing agents than is triple negative GBC.
To investigate the effects of hormones in more detail, we selected ER-, AR-, and HER/neu-positive GBC and assayed procaspase-3 and active caspase-3 by Western blotting. ER-positive GBC showed significant expression of active caspase-3, indicating that the ER-positive cases were more susceptible to apoptosis and had a high rate of internal apoptosis. Supplementary induction of apoptosis by chemotherapeutic agents may thus accelerate its effect. HER/neu-positive GBC also exhibited high active caspase-3 expression. However, the AR-positive GBC results were interesting, in terms of the increased levels of active procaspase-3 and less active caspase-3 expression, compared to ER- and HER/neu-positive GBC. Overall, the level of caspase-3 protein was lowest among other hormone-responsive GBC, with AR being the least sensitive to apoptosis.
In conclusion, our findings reflect the status of apoptosis in GBC, and indicated that ER- and HER/neu-positive GBC tumors are highly sensitive to apoptosis and may respond better to chemotherapeutic drugs such as tamoxifen, whereas AR-positive GBC is more resistant to apoptosis and hence requires more aggressive treatment than ER- and HER/neu-positive GBC.
KEY MESSAGE
1. HER and estrogen receptor (ER) positive gallbladder tumors have more sensitivity for apoptosis compare to androgen receptor (AR) positive gallbladder tumors.
2. HER and ER positive gallbladder tumor may give better chemotherapy response in comparison to AR positive gallbladder tumor with apoptotic inducing drugs.
3. AR positive gallbladder cancer may require more aggressive treatment compare to HER and ER positive gallbladder cancer.
Acknowledgments
Authors would like to thank University Grant Commission, New Delhi, India for financial support.
Notes
No potential conflict of interest relevant to this article is reported.