Third-line docetaxel chemotherapy for recurrent and metastatic gastric cancer
Article information
Abstract
Background/Aims
To determine the efficacy and toxicity of docetaxel as a third-line therapy for patients with relapsed gastric cancer who have undergone modified oxaliplatin-fluorouracil (m-FOLFOX)-4 and modified irinotecan-fluorouracil (m-FOLFIRI) regimens.
Methods
We analyzed 33 patients who had been histologically diagnosed with adenocarcinoma of the stomach and who had progressed after m-FOLFOX-4 and m-FOLFIRI regimens. Patients were treated with cycles of 75 mg/m2 docetaxel on day 1 every 3 weeks.
Results
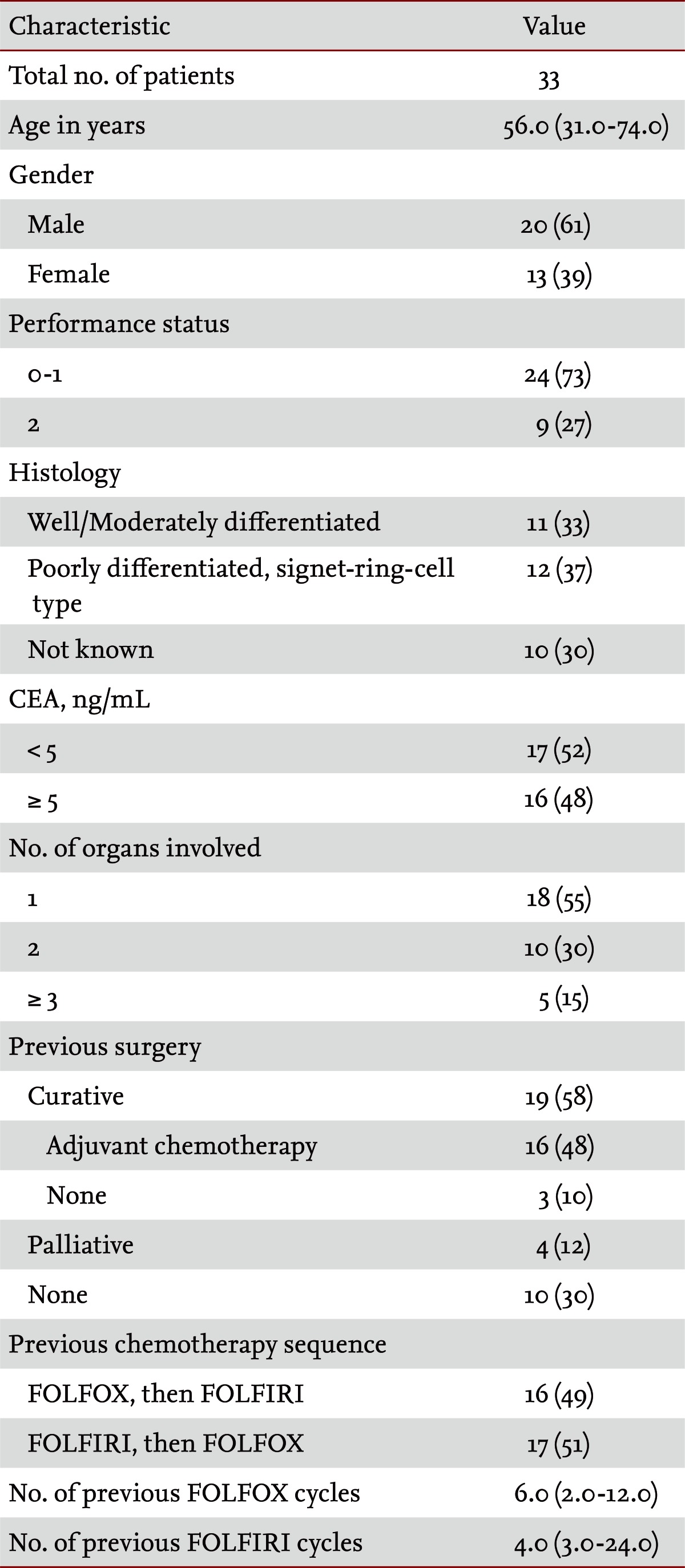
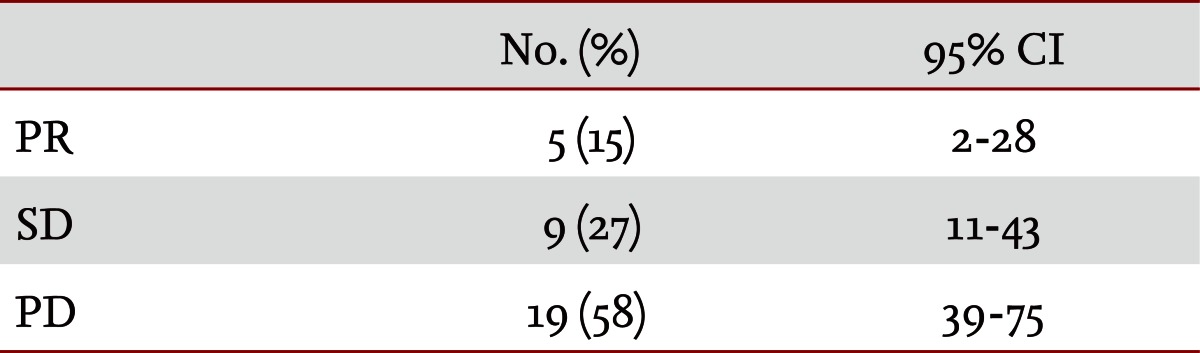
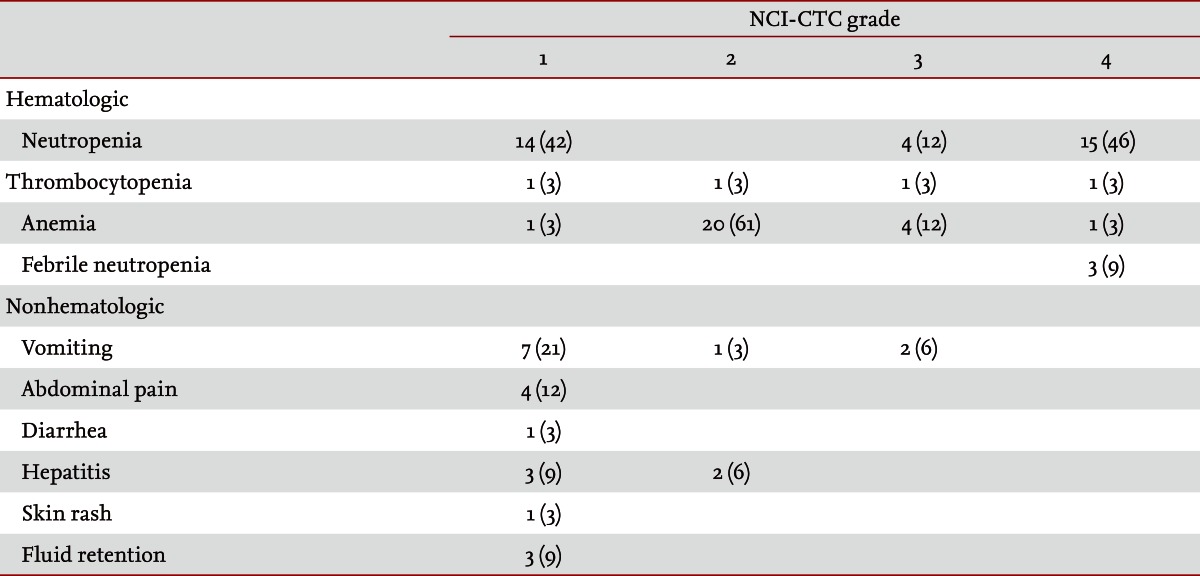
The median age of the patients was 56.0 years (range, 31.0 to 74.0), and 73% of the patients (24/33) had an Eastern Cooperative Oncology Group performance status of 0 or 1. All patients were evaluated in terms of tumor response: five (15%), nine (27%), and 19 (58%) patients experienced a partial response, stable disease, and progressive disease, respectively. The median time to progression was 2.1 months (95% confidence interval [CI], 1.63 to 2.58), and overall survival was 4.7 months (95% CI, 3.20 to 6.20), from the start of the docetaxel regimen. Assessing patients' toxicity profiles, the median number of cycles was 2.0 (range, 1.0 to 12.0). The major hematologic toxicities included grade 3 to 4 neutropenia (19/33, 58%), grade 3 to 4 thrombocytopenia (2/33, 6%), and grade 3 to 4 anemia (5/33, 15%). Neutropenic fever developed in three patients (3/33, 9%). The nonhematological toxicities were nausea and vomiting (10/33, 30%), abdominal pain (4/33, 12%), skin rash (1/33, 3%), and fluid retention (3/33, 9%).
Conclusions
Docetaxel is a feasible third-line therapy regimen for patients with advanced gastric cancer after m-FOLFIRI and m-FOLFOX-4 regimens.
INTRODUCTION
Gastric cancer is ranked as the second leading cause of cancer-related death globally [1]. Most gastric cancer patients are diagnosed at an advanced stage or progress after curative surgical resection. In this clinical context, aside from supportive care, chemotherapy is the only treatment option. Single or combination chemotherapy regimens have proven to improve survivorship 3-fold, compared with the best supportive care possibilities [2-4]. Several cytotoxic chemotherapy agents, including fluorouracil, platinum compounds, taxanes, irinotecan, and anthracyclines, showed modest activity in gastric cancer, with response rates (RRs) ranging from 10% to 25%, but no single drug has been proven to have greater efficacy compared to other agents.
Docetaxel is a second-generation taxane. A semisynthetic analogue of paclitaxel, it binds to and stabilizes tubulin, preventing physiological microtubule polymerization and disassembly, and thereby blocking the cell cycle at the metaphase/anaphase transition and inhibiting cell proliferation [5,6]. The mechanism of action of docetaxel is distinct from those of irinotecan, oxaliplatin and fluorouracil, and it has little cross-resistance with these widely accepted chemotherapy agents.
Docetaxel has been included in many clinical trials as a single agent or in combination chemotherapy regimens. Docetaxel as a single regimen was administered at 60 to 100 mg/m2 every 3 weeks. In chemotherapy-naïve patients, the overall RR was 17% to 24%, while in the salvage setting, the overall RR was 4.8% to 22%. RRs of 22% and 20% were achieved at with does of 60 and 100 mg/m2, respectively. The most common adverse events were neutropenia, stomatitis, nausea, vomiting, and diarrhea. Less commonly, patients suffered from fluid retention and hypersensitivity [7-12].
Several studies have reported the efficacy of third-line chemotherapy in terms of RR and overall survival (OS), but there is still no established third-line chemotherapy regimen for metastatic or recurrent gastric cancer. We thus conducted a study of 3-week docetaxel monotherapy in advanced gastric cancer patients who had failed to respond to both oxaliplatin with low-dose leucovorin (ldLV) plus bolus and continuous infusion 5-fluorouracil (5-FU; modified oxaliplatin-fluorouracil-4 [m-FOLFOX-4]) and irinotecan plus ldLV and bolus and continuous infusion 5-fluorouracil (modified irinotecan-fluorouracil [m-FOLFIRI]). We evaluated the efficacy and toxicity of the regimen.
METHODS
Patients
Thirty-three patients received docetaxel monotherapy as third-line chemotherapy at the Department of Internal Medicine of the Dong-A Medical Center in Busan, South Korea, between July 2006 and July 2011. This retrospective study included patients who: 1) had histologically confirmed adenocarcinoma of the stomach; 2) had metastatic disease at initial diagnosis or progressive disease (PD) after curative surgery; 3) failed to achieve a response to both m-FOLFOX-4 and m-FOLFIRI regimens, with the sequence of these regimen being interchangeable; 4) were aged above 18 years; 5) had no central nervous system metastases; 6) had no active infection; 7) had no serious or uncontrolled concurrent medical illness; 8) had no history of other malignancies; 9) had sufficient hepatic (transaminase level ≤ 2.5 times the normal value), renal (serum creatinine concentration ≤ 1.5 mg/dL and blood urea nitrogen level ≤ 50 mg/dL), and bone marrow functions (white blood cells ≥ 3,000/mm3, hemoglobin ≥ 10 g/dL, and platelets ≥ 70,000/mm3); and 10) had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2. This study was approved by the appropriate institutional review board, and all patients gave written informed consent (IRB no. 11-147).
Treatment protocol and toxicity assessment
Chemotherapy was administered through a chemoport positioned in the right subclavian vein or directly into a peripheral vein. On day 1, docetaxel (75 mg/m2) was administered by intravenous infusion in 500 mL of dextrose over 1 hour in a 3-week cycle. Dose modifications of docetaxel were made on the basis of hematologic toxicities, considering the most severe grade of toxicity that had occurred during the previous cycle. Toxicities were assessed prior to the initiation of each 3-week cycle using the Common Terminology Criteria for Adverse Events Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Treatment was delayed for up to 1 week if toxicity persisted; i.e., when the absolute neutrophil number was ≤ 1,500/mL or the platelet count was ≤ 100,000/mL. The dosage of docetaxel was reduced by 25% in subsequent courses when CTCAE grade 4 neutropenia, neutropenic fever or thrombocytopenia, or grade 3 diarrhea and/or hepatotoxicity, occurred. Treatment was continued until signs of disease progression or unacceptable toxic effects developed, or until the patient refused further treatment.
Follow-up evaluation and assessment of response
Prior to each course of treatment, a physical examination, routine hematologic studies, blood chemistry tests, and chest X-rays were conducted. Computed tomography scans were performed to define the extent of disease and response after 2 to 3 cycles of chemotherapy, or sooner in cases in which there was evidence of clinical deterioration.
Responses of measurable lesions were evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.0) [13]. Complete response (CR) was defined as the disappearance of all evidence of disease and the normalization of tumor markers for at least 2 weeks. Partial response (PR) was defined as a 30% reduction in unidimensional tumor measurements, without the appearance of any new lesions or the progression of any existing lesion. PD was defined as any of the following: 1) a 20% increase in the sum of the products of all measurable lesions; 2) the appearance of any new lesion; or 3) the reappearance of any lesion that had previously disappeared. Stable disease (SD) was defined as a tumor response not fulfilling the criteria for CR, PR or PD.
Dose intensity (mg/m2/wk) was calculated as the total cumulative dose divided by the duration of treatment. Relative dose intensity (RDI) was calculated by dividing the dose intensity by the planned dose intensity, and was expressed as a percentage.
Endpoints and statistical analysis
The primary endpoint of this study was RR, and the secondary endpoints were time to progression (TTP), OS, and treatment toxicities. RRs with regard to the different variables were compared using Fisher's exact test. TTP and OS were calculated using the Kaplan-Meier method. TTP was calculated from the date at which therapy was initiated to the date of disease progression for patients who discontinued therapy due to disease progression or for reasons other than disease progression. OS was calculated from the date at which therapy was initiated to the date of death or final follow-up. Prognostic factors for response, TTP and OS were analyzed using Fisher's exact test, the Pearson's chi-squared test, the Kaplan-Meier estimator and Cox regression analysis. All data were analyzed using the IBM SPSS software version 19.0 (IBM Co., Armonk, NY, USA).
RESULTS
Patient characteristics
Patients' baseline characteristics are shown in Table 1. The median age of the patients was 56.0 years (range, 31.0 to 74.0), the male to female ratio was 20 : 13 and 73% of the patients (24/33) had an ECOG performance status of 0 or 1. Nineteen patients underwent curative surgery, of whom 16 received adjuvant chemotherapy. Eighteen patients (55%) showed involvement of one organ at the time of docetaxel chemotherapy; the most frequent site of metastasis was the peritoneum (16 patients). Sixteen (49%) and 17 (51%) patients, respectively, underwent sequential FOLFOX-FOLFIRI and FOLFIRI-FOLFOX regimens. The median numbers of cycles of m-FOLFOX-4 and m-FOLFIRI regimens were 6.0 (range, 2.0 to 12.0) and 4.0 (range, 3.0 to 24.0), respectively.
Response
A total of 33 patients were assessed for response in terms of measurable lesions. Twenty patients initially evidenced measurable lesions and five patients developed new lesions. Five patients (15%) experienced a PR, nine (27%) had SD and PD occurred in 19 patients (58%) (Table 2). The mean RR was 15% (95% confidence interval [CI], 5.1 to 31.9).
TTP and OS
The median TTP was 2.1 months (95% CI, 1.63 to 2.58), and the median OS duration 4.7 months (95% CI, 3.20 to 6.20) from the start of the docetaxel regimen. TTP and OS were evaluated by Kaplan-Meier analysis, the results of which are shown in Figs. 1 and 2. Early death (within 30 days of treatment) was observed in one patient and was related to disease progression. Univariate and multivariate analyses of the prognostic factors affecting TTP and OS showed no significant relationships with age, gender, performance status, carcinoembryonic antigen level, the sequence of FOLFOX and FOLFIRI regimens or the response to FOLFOX and FOLFIRI regimens.
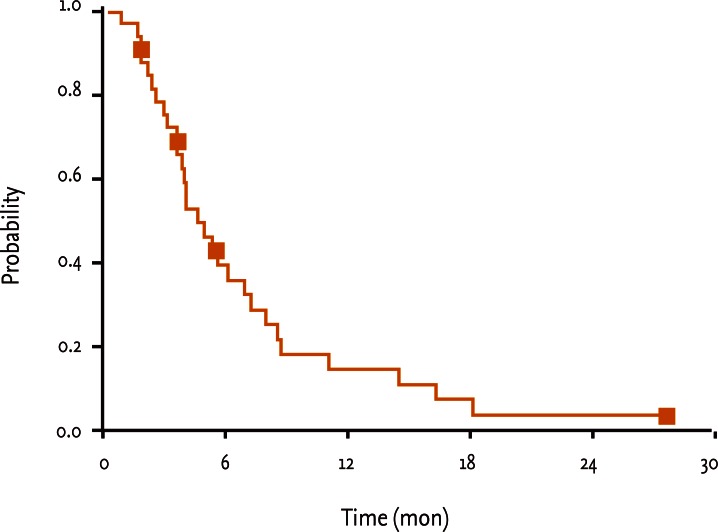
Overall survival rates. The median overall survival duration from the start of the docetaxel regimen was 4.7 months (95% confidence interval, 3.20 to 6.20).
Toxicity and dose administration
The median number of cycles of docetaxel treatment was 2.0 (range, 1.0 to 12.0). Dose reductions were required on 19 occasions. The median dose intensity of docetaxel was 20.3 mg/m2/wk (range, 17 to 25) and the median RDI was 0.8 (range, 0.7 to 1.0). The most common grade 3 or 4 hematologic toxicity event was neutropenia (19/33, 58%), followed by anemia (5/33, 15%), and thrombocytopenia (2/33, 6%). Neutropenic fever developed in three patients (3/33, 9%). Grade 1/2 nausea and vomiting were observed in eight patients (8/33, 24%), and two patients (2/33, 6%) suffered from grade 3 nausea and vomiting. Grade 1 abdominal pain occurred in four patients (4/33, 12%), grade 1/2 hepatitis was observed in five patients (5/33, 15%), one patient (1/33, 3%) experienced a skin rash and fluid retention was noted in three patients (3/33, 9%) (Table 3). Most of the patients discontinued treatment because of disease progression. Among the six patients who stopped treatment for reasons other than disease progression, four did so because of treatment toxicities: pneumonia in one case, grade 4 neutropenia combined with infection in one case and general weakness in two cases. Two other patients ceased treatment due to cancer-related gastrointestinal bleeding and postoperative adhesive ileus. No serious complications that required aggressive supportive care and no treatment-related deaths occurred in this study.
DISCUSSION
Multiple combinations of chemotherapeutic agents can offer the best palliation in terms of prolonged survival, symptom control, and improved quality of life in recurrent and metastatic gastric cancer. Although many active agents have been studied, no first-, second-, or third-line regimens have been established to date, as most trials have resulted in no superior efficacies being found.
Oxaliplatin and irinotecan have been proven in many clinical trials to be effective chemotherapeutic agents when combined with 5-FU in recurrent or metastatic gastric cancer as first- or second-line treatment. They have also been proven to have acceptable toxicity profiles. Previous prospective studies have proved the efficacy and tolerability of m-FOLFIRI and m-FOLFOX-4 regimens in recurrent or metastatic gastric cancer as both first- and second-line chemotherapies. As a first-line regimen for recurrent/metastatic gastric cancer, m-FOLFIRI showed an overall RR of 38.6% and an OS of 10.3 months [14]. When m-FOLFIRI was administered as a salvage regimen in gastric cancer patients, they showed an overall RR of 10.0% and an OS time of 10.9 months [15]. Advanced gastric cancer patients who were treated with an m-FOLFOX-4 regimen as first-line chemotherapy showed an overall RR of 50% and an OS time of 7.7 months [16]. As salvage chemotherapy in gastric cancer patients, the m-FOLFOX-4 regimen was found to have an overall RR of 26.7% and an OS time of 7.9 months [17]. Based on these studies, we administered m-FOLFOX-4 and m-FOLFIRI regimens as first- or second-line chemotherapies. The sequence of the two regimens was interchangeable.
Several studies have proved that second- and third-line chemotherapy performs better for advanced gastric cancer, compared with the best supportive care methods, in terms of OS and quality of life. Commonly adopted drugs in these trials were taxanes [18-20].
Our study analyzed patients who had failed to respond to m-FOLFOX-4 or m-FOLFIRI regimens, and who were thus treated with docetaxel monotherapy as a third-line regimen. Because docetaxel has a different mechanism of action than topoisomerase inhibitors, alkylating agents, and antimetabolites, the idea of introducing this second-generation taxoid into secondline treatment of advanced gastric cancer has been proposed. Several phase II trials have demonstrated the efficacy of docetaxel monotherapy for recurrent and metastatic gastric cancer. A study of 49 patients who failed to respond to 5-FU/platinum combination chemotherapy reported a RR of 16.3% (95% CI, 6.0 to 26.6) and median OS of 8.3 months (95% CI, 6.7 to 9.8) with a dose of 75 mg/m2 [11]. A multicenter Italian study evaluated 30 patients who were refractory to first-line epirubicin/cisplatin/5-FU or cisplatin/epirubicin/leucovorin/5-FU regimens and reported a RR of 17% (95% CI, 6 to 36) and median OS of 6.0 months with a dosage of 100 mg/m2 [9]. An early multicenter Japanese study analyzed 59 patients and reported a RR of 23.7% (95% CI, 13.6 to 36.6) with a dose of 60 mg/m2 [12]. In this study, we administered docetaxel at a dose of 75 mg/m2 at 3-week intervals to patients who were resistant to both m-FOLFOX-4 and m-FOLFIRI regimens.
Of the 33 patients, 15% (5/33) showed responses, with an overall disease control rate of 42%. The median TTP was 2.1 months (95% CI, 1.63 to 2.58) and the median OS was 4.7 months (95% CI, 3.20 to 6.20) from the start of the docetaxel regimen. No randomized or retrospective studies conducted using a similar setting to our own have compared the efficacy of third-line chemotherapy. The RR, TTP, and OS values in this study are lower than those found in previous second-line treatment studies of refractory gastric cancer. A lower efficacy of third-line chemotherapy in multi-agent resistant gastric cancer was also reported in a retrospective single-center study of 532 patients [18]. Among the 460 patients who received chemotherapy, 23% underwent third-line chemotherapy, showing an RR of 10.9%, a disease control rate of 54.2%, median progression free survival of 2.5 months, and median OS of 5.5 months from the start of any third-line chemotherapy, regardless of their first- or second-line regimens. The efficacy of chemotherapy dropped as the disease progressed after each chemotherapeutic regimen. However, the OS rate was 12.1 months in the chemotherapy group versus 2.5 months in the supportive care group. In this study, the average time from cancer recurrence or diagnosis of metastasis to death was 12.8 months (95% CI, 9.16 to 18.44).
The lowering of the RR as chemotherapies proceed can be explained by the expansion of resistant clones, the increased tumor burden and a decrease in drug dosage compared to previous chemotherapeutic agents because of the clinician's concern about the patient's intolerance to drugs. However, there is no doubt that chemotherapy can increase OS in recurrent or metastatic gastric cancer patients when compared to the best supportive care available. Previous studies reported that performance status and metastatic patterns are consistent prognostic factors throughout salvage chemotherapy. In our study, however, using Cox regression analysis, we found no factors affecting TTP and OS. The small number of patients analyzed and the nature of the disease itself may have skewed the prognostic factor analysis.
The most common hematologic toxicity due to docetaxel in this study was grade 3 to 4 neutropenia (58%), followed by anemia (15%) and thrombocytopenia (9%). Neutropenic fever developed in two patients (6%) on days 7 to 9, but there were no serious infections that required intensive antibiotic therapy. The most common nonhematological toxicities were nausea and vomiting, hepatotoxicity, abdominal pain, fluid retention, and skin rash. Although the patients had previously been heavily treated with two regimens, most of them tolerated docetaxel relatively well. Only two patients died of rapid disease progression after one cycle of docetaxel chemotherapy, and no life-threatening complications due to docetaxel were observed. Contrary to concerns about the life-threatening toxicity of third-line chemotherapeutic treatments for multi-agent-refractory gastric cancer patients, most of our patients tolerated docetaxel chemotherapy reasonably well.
This study has certain limitations. First, too few patients were analyzed to form general conclusions. Second, this study was retrospective in nature. Third, although all of the patients had been treated with m-FOLFOX-4 and m-FOLFIRI regimens, the sequence of, or previous responses to, first- and second-line regimens may have influenced the third-line docetaxel treatment results. And fourth, more than half of the patients required dose reductions, suggesting that the effect of docetaxel was diminished and possibly masking the toxicity of the drug. The distribution of patients who received FOLFOX-FOLFIRI and FOLFIRI-FOLFOX chemotherapies in this study was even (49% and 51%, respectively) and in univariate and multivariate analyses, the sequence of previous chemotherapeutic regimens and the maximal response to m-FOLFOX-4 and m-FOLFIRI regimens did not affect the RR, TTP, and OS for docetaxel chemotherapy. Despite the number of patients being small and the study being retrospective, this is the only study of patients who were resistant to all three of the most commonly prescribed chemotherapeutic drugs (oxaliplatin, irinotecan, and fluorouracil). Over half of the patients in this study received less than the planned dosage; however, the median RDI was 0.8, which is thought to be acceptable in the real clinical setting. This study could therefore reflect routine clinical practice and could be developed into a more systemized trial.
In conclusion, our data suggest that, as a single regimen, docetaxel is well tolerated and could be an option for patients who are resistant to fluorouracil, oxaliplatin, and irinotecan. Randomized comparisons in a large cohort between third-line chemotherapy-treated patients and those who receive best supportive care after failure of the same active agents in first- and second-line chemotherapies are needed to establish effective third-line regimens in patients with multiagent-refractory gastric cancer.
KEY MESSAGE
1. Salvage chemotherapy in advanced gastric cancer patients who failed first- or second-line chemotherapy can improve survival.
2. Three-week cycle docetaxel monotherapy was beneficial in advanced gastric cancer patients who have progressed after modified oxaliplatin-fluorouracil-4 and modified irinotecan-fluorouracil regimens.
3. Toxicity profile of docetaxel in salvage setting was acceptable.
Acknowledgments
This paper was supported by the Dong-A University Research Fund.
Notes
No potential conflict of interest relevant to this article is reported.