Is the frequency of metabolic syndrome higher in South Korean women with rheumatoid arthritis than in healthy subjects?
Article information
Abstract
Background/Aims
To compare the frequency of metabolic syndrome (MetS) and magnitude of insulin resistance, measured by the homeostatic model assessment of insulin resistance (HOMA-IR), between South Korean women with rheumatoid arthritis (RA) and healthy subjects, and to evaluate risk factors for MetS and increased HOMA-IR in patients with RA.
Methods
In a cross-sectional setting, 84 female patients with RA and 109 age-matched healthy female subjects were consecutively recruited at a university-affiliated rheumatology center in South Korea. MetS was defined according to the Third Report of the National Cholesterol Education Program's Adult Treatment Panel (NCEP-ATP III) 2004 criteria.
Results
The frequency of MetS did not differ significantly between patients with RA (19%) and healthy subjects (15.6%, p = 0.566), although patients with RA had a higher HOMA-IR compared with healthy subjects (p < 0.001). Patients with RA met the NCEP-ATP III 2004 criteria for high blood pressure more often than healthy subjects (44% vs. 19.3%, p < 0.001), and low high density lipoprotein cholesterol was more prevalent in healthy subjects (33%) than in patients with RA (14.3%, p = 0.004). Although no obvious risk factors for the presence of MetS were identified in patients with RA, higher serum C-reactive protein and disease activity score assessed using the 28-joint count for swelling and tenderness-erythrocyte sedimentation rate significantly contributed to a higher HOMA-IR.
Conclusions
Despite their increased insulin resistance, South Korean women with RA did not have a significantly higher frequency of MetS compared with that in healthy subjects.
INTRODUCTION
Metabolic syndrome (MetS), also known as syndrome X or insulin resistance syndrome, comprises obesity, insulin resistance, impaired glucose tolerance or diabetes, hypertension, and dyslipidemia, all of which are known risk factors for atherosclerosis [1]. Among these factors, insulin resistance is recognized as the key pathophysiological factor for MetS. Moreover, insulin resistance per se increases the risk for cardiovascular diseases (CVDs) and contributes to the association between MetS and coronary atherosclerosis [2,3]. Although the value of MetS as a predictor of cardiovascular risk has been much debated, a recent meta-analysis showed that MetS is associated with a 2-fold increase in cardiovascular outcome and a 1.5-fold increase in all-cause mortality [4]. Hence, MetS has grown in importance in light of its contribution to the burden of cardiovascular morbidity and mortality in recent years.
Recent studies have demonstrated that in addition to insulin resistance, inflammation is closely associated with the pathogenesis of MetS [1,5,6]. A rise in acutephase reactants such as C-reactive protein (CRP) and proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), promote insulin resistance [1,7,8]. Inflammatory biomarkers are frequently elevated in subjects with MetS, and conversely, MetS is prevalent in patients with chronic inflammatory status such as rheumatic diseases [6].
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease characterized by articular and extra-articular involvement. Patients with RA have an increased risk for CVDs due to accelerated atherosclerosis as a result of both increased inflammatory cytokines and a higher prevalence of traditional risk factors such as type 2 diabetes mellitus (DM) and hypertension [9,10]. MetS may provide an additional connection between accelerated atherosclerosis and inflammation in RA [7]. MetS is a common manifestation in patients with RA, but previously reported frequencies of MetS among patients with RA vary widely, from 14% to 56% [11-24]. This diversity may be attributable to differences in the definition of MetS, ethnicity, geographic area, study design, and study population characteristics. Moreover, some studies have demonstrated a higher prevalence of MetS in patients with RA than in the general population [14,19,21], whereas others have not [12,13,22-24]. This discrepancy warrants further exploration. In addition, the prevalence of MetS in South Korean women with RA has not been investigated to date.
The objectives of the present study were to compare the frequency of MetS between South Korean female patients with RA and healthy subjects and to evaluate factors associated with the presence of MetS in patients with RA. Additionally, insulin resistance was measured by the homeostatic model assessment of insulin resistance (HOMA-IR) and compared between patients with RA and healthy subjects.
METHODS
Study design and subjects
We designed a cross-sectional study that included 84 female patients with RA and 109 age-matched female healthy subjects (± 2 years) (age range, 22 to 76). The entire study population was consecutively recruited at a university-affiliated rheumatology center in South Korea from January 2008 to December 2009. All patients with RA fulfilled the American College of Rheumatology 1987 revised classification criteria for RA [25]. Patients with rheumatic diseases other than RA or who refused to participate in this study were excluded. Healthy subjects were selected randomly from among applicants undergoing an annual health check in the same center and had no history of taking any medications such as glucocorticoids (GCs) or oral contraceptives that would affect insulin resistance and no current autoimmune or rheumatic diseases. Written informed consent was obtained from each subject based on the Declaration of Helsinki. This study was approved by the Research and Ethics Review Board of the Pusan National University Hospital, Busan, South Korea.
Assessments
All information was collected during an interview and by reviewing medical records. Anthropometric parameters, including height, weight, body mass index (BMI), waist circumference, and blood pressure, were measured in all study subjects. BMI was calculated by dividing body weight by the square of height in meters (kg/m2), and waist circumference was measured using a tape at the midpoint between the lower part of the lowest rib and the highest point of the iliac crest on the mid-axillary line. Blood pressure was determined as the mean of two measurements taken at an interval of 5 minutes using a TM-2655P apparatus (A&D Company Ltd., Tokyo, Japan). Hypertension was defined as blood pressure ≥ 140/90 mmHg or requiring antihypertensive medication.
Study subjects also underwent biochemical assessments. Fasting blood samples of all participants were taken between 8:00 AM and 10:00 AM to determine total cholesterol (TC), triglycerides (TGs), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), fasting glucose and insulin, and CRP. The concentrations of TC, TGs, and HDL-C were analyzed using an enzymatic colorimetric reagent (Roche Diagnostics, Zurich, Switzerland) and a P800 Module (Roche Diagnostics). LDL-C was calculated using the Friedewald formula. CRP was measured with a particle-enhanced immunoturbidimetric assay (Tina-quant C-reactive protein assay, Roche Diagnostics) using a P800 Module (Roche Diagnostics). Fasting glucose and insulin were assessed by the glucose oxidase method (Synchron LX-20, Beckman Coulter Inc., Fullerton, CA, USA) and radioimmunoassay (Diagnostic Product Co., Los Angeles, CA, USA), respectively.
The following additional data were collected for patients with RA: disease duration, medication records, erythrocyte sedimentation rate (ESR; mm/hr), CRP (mg/dL), immunoglobulin M-rheumatoid factor (RF; IU/mL), and disease activity score assessed using the 28-joint count for swelling and tenderness (DAS28)-ESR. Medication records included the use of GCs; disease modifying antirheumatic drugs (DMARDs), including methotrexate, hydroxychloroquine, sulfasalazine, leflunomide, and tacrolimus; TNF inhibitors, and antihypertensive drugs. We calculated the cumulative GC dose by multiplying the current daily dose by the number of days the patient had been taking GCs since they were first prescribed. RF was assessed by particle enhanced immunoturbidometric assay, and seropositivity was defined as > 14.0 IU/mL. DAS28-ESR was calculated using the following formula [26]:
MetS was defined according to the Third Report of the National Cholesterol Education Program's Adult Treatment Panel (NCEP-ATP III) 2004 [27], using the Asian criteria for central obesity [28] when three or more of the following components were present: 1) waist circumference ≥ 80 cm in women, 2) elevated blood pressure ≥ 130/85 mmHg or requiring drug therapy, 3) elevated serum TG level ≥ 150 mg/dL, 4) reduced serum HDL-C ≤ 50 mg/dL in women, and 5) elevated fasting glucose level ≥ 100 mg/dL or requiring drug therapy. Insulin resistance was evaluated by HOMA-IR, which was calculated with the formula defined by Matthews et al. [29] as follows:
Statistical analysis
Data are summarized as mean (standard deviation) or median (interquartile) for continuous variables and as number (percentage) for categorical variables. The Kolmogorov-Smirnov test was used to assess the distributions of continuous variables. The two-tailed Student's t test or Mann-Whitney U test was used to compare continuous variables between patients with RA and healthy subjects, and the chi-squared test or Fisher's exact test was performed for categorical variables. Univariate and multivariate logistic regression models were used to estimate unadjusted and adjusted odds ratios (ORs) for factors associated with the presence of MetS. The multivariate logistic regression model included variables with p < 0.20 in the univariate logistic regression analysis. To approximate a normal distribution, natural log-transformed HOMA-IR values were used in Pearson's correlation analysis and stepwise multivariate linear regression analysis for estimating independent predictors of increased insulin resistance. Values of p < 0.05 were considered to indicate statistical significance. All statistical analyses were carried out using STATA version 11.1 for Windows (StataCorp LP, College Station, TX, USA).
RESULTS
Clinical characteristics of the study subjects
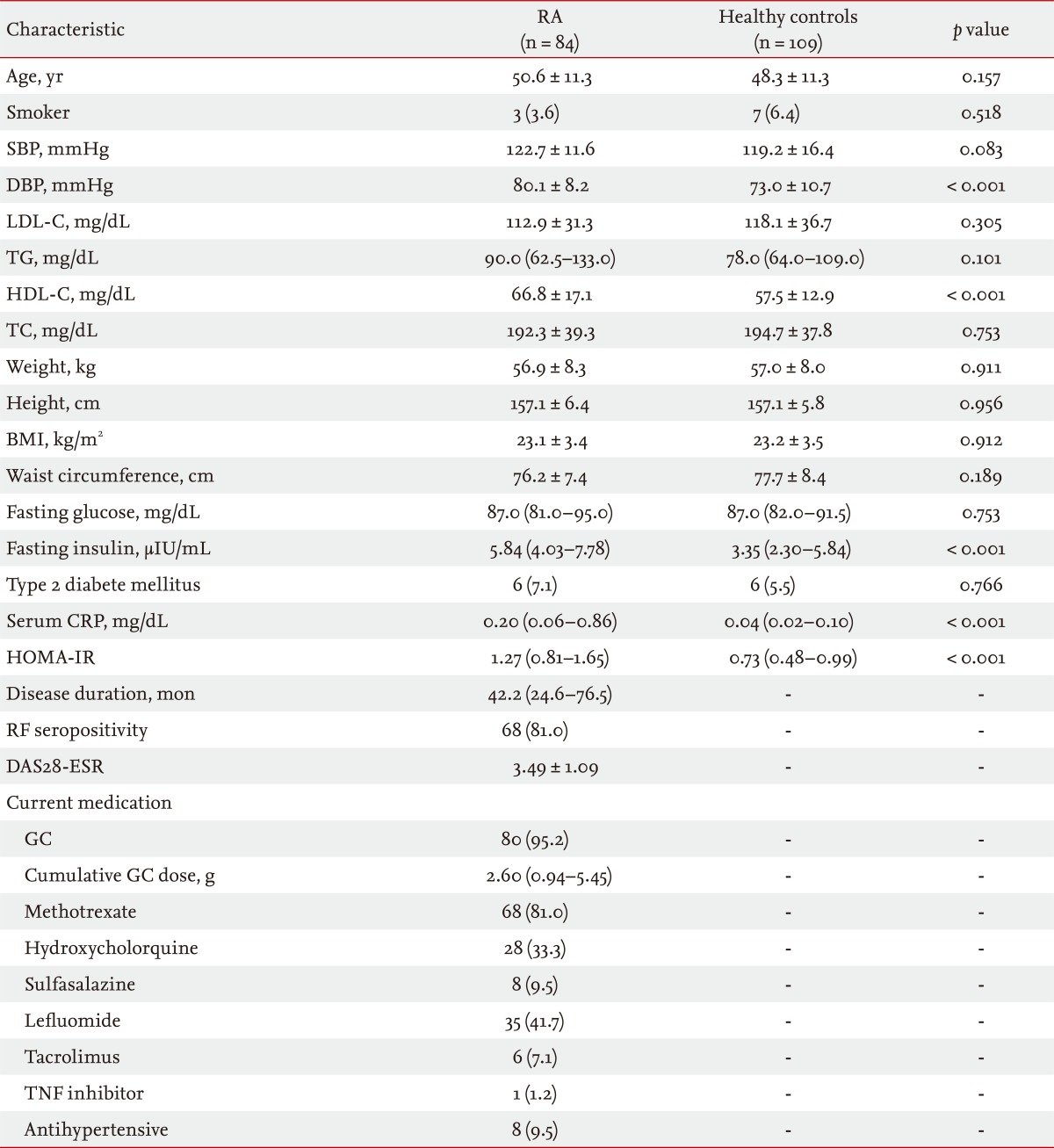
The demographics of the study subjects are summarized in Table 1. The mean ± SD age of the 84 female patients with RA was 50.6 ± 11.3 years, and the median (interquartile range) disease duration was 42.2 (24.6 to 76.5) months. Sixty-eight patients (81%) had RF seropositivity, and the mean ± SD DAS28-ESR in patients with RA was 3.49 ± 1.09. The majority of patients were taking GCs, and all but one of the patients with RA was treated with at least one DMARD. Compared with the healthy subjects, patients with RA had significantly higher diastolic blood pressure, HDL-C, fasting serum insulin, and serum CRP. No significant differences were observed according to age, proportion of smokers, systolic blood pressure, LDL-C, TG, BMI, waist circumference, fasting serum glucose, or percentage with type 2 DM between the two groups. HOMA-IR was significantly higher in patients with RA than in healthy subjects (p < 0.001), suggesting that patients with RA were more insulin resistant than healthy subjects.
Frequency of MetS and MetS-related features
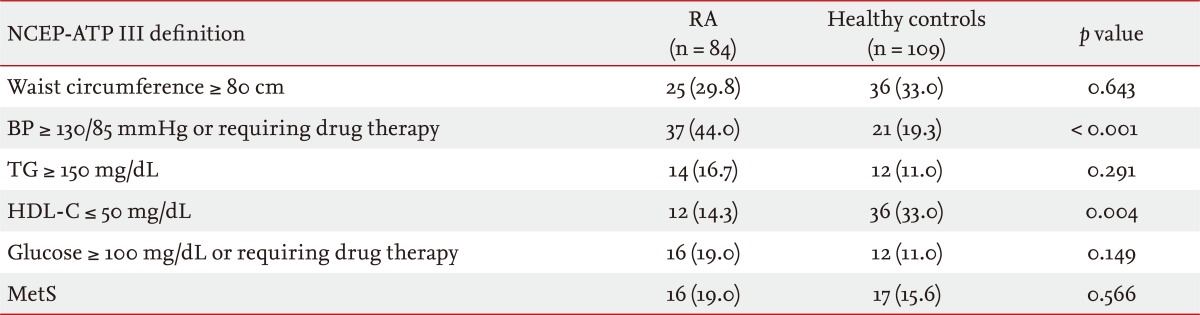
Table 2 shows the frequency of the individual MetS criteria, according to the NCEP-ATP III 2004, in the study population. The frequency of MetS in patients with RA (19%) was not significantly higher than that in healthy subjects (15.6%, p = 0.566). Patients with RA met the NCEP-ATP III 2004 criteria for high blood pressure more often than healthy subjects (44% vs. 19.3%, p < 0.001), and low HDL-C was more prevalent in healthy controls than in patients with RA (33% vs. 14.3%, p = 0.004). No significant differences in waist circumstance, TG, or glucose criteria were seen between the two groups.
Factors associated with MetS and increased insulin resistance
Table 3 shows the ORs of disease-related variables for the presence of MetS in 84 female patients with RA. In the univariate analyses, higher age (OR, 1.08; 95% confidence interval [CI], 1.02 to 1.14) and longer disease duration (OR, 1.01; 95% CI, 1.00 to 1.12) were related to an increased frequency of MetS, whereas serum CRP, RF seropositivity, DAS28-ESR, cumulative GC dose, and hydroxychloroquine and methotrexate use did not demonstrate a significant association. Longer disease duration tended to be associated with MetS in the multivariate logistic regression model, but did not remain statistically significant after adjusting for age (p = 0.084). To identify an independent relationship between disease duration and MetS, we entered disease duration as a dichotomous variable, instead of a continuous variable, using the cutoff point defined by the median value (< 42, ≥ 42 months) in the multivariate model; age was still not statistically significant (data not shown).
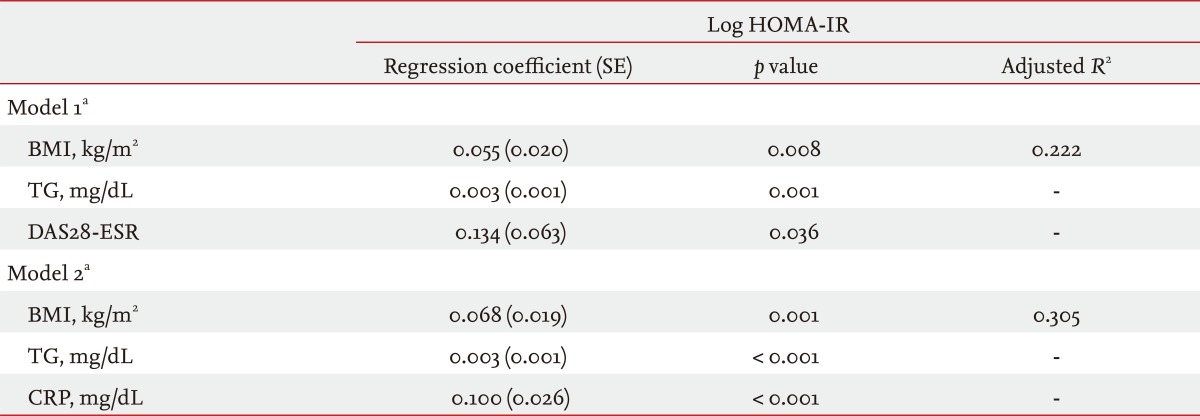
Because HOMA-IR was not normally distributed, we used natural log-transformed HOMA-IR values to assess the risk factors for insulin resistance. In the Pearson's correlation analyses, age, TG, BMI, waist circumference, serum CRP, and DAS28-ESR were positively correlated with log HOMA-IR (data not shown). Whether RA-specific variables such as serum CRP and DAS28-ESR are independent predictors for insulin resistance was of interest. However, the two variables were significantly correlated (p = 0.010). Two stepwise multivariate linear regression models that included CRP and DAS28-ESR were constructed separately to avoid multicollinearity (Table 4). Both RA specific variables remained significant, and the magnitude of the statistical association in the multivariate models was greater for CRP than for DAS28-ESR (p < 0.001 and p = 0.036, respectively). Higher TG and BMI were also independent risk factors for log HOMA-IR, but age was not.
DISCUSSION
In the present study, no significant difference was observed in the frequency of MetS between female patients with RA and healthy subjects, whereas the magnitude of insulin resistance in patients with RA was significantly higher than that in healthy subjects. Despite the lack of obvious risk factors for the presence of MetS in patients with RA, RA inflammation (CRP), and disease activity (DAS28-ESR) significantly contributed to increased insulin resistance as measured by HOMA-IR.
A growing body of evidence indicates that both MetS and RA are closely related to increased cardiovascular morbidity and mortality, which has led to the need to evaluate whether MetS is more prevalent in patients with RA compared with healthy subjects in the last decade. Several studies have demonstrated a higher prevalence in patients with RA than in healthy controls [14,19,21], whereas others have not [12,13,22-24]. Our results yielded no difference in the frequency of MetS between patients with RA and healthy subjects. Interestingly, Sahebari et al. [23] reported that the frequency of MetS in patients with RA was significantly lower than that in healthy controls. The epidemiological association between RA and MetS has not yet been fully determined, and various factors may be responsible for the differences in the prevalence of MetS in patients with RA. Among these factors, ethnicity and geographic area appeared to affect the difference in MetS between patients with RA and healthy controls. Two of three previous studies in Asian subjects reported a similar prevalence of MetS in patients with RA than in controls [23,24]. Considering that our study included South Korean subjects only, it is presumed that MetS tends to be less prevalent among Asian patients with RA. This assumption was also suggested in a recent systematic review by Yiu et al. [30]. As the number of studies is limited, further research is needed to confirm the relationship between RA and MetS and to evaluate the effect of ethnicity and geographic area on the frequency of MetS.
No significant risk factors for MetS were observed in female patients with RA. In some studies, methotrexate therapy was associated with reduced prevalence of MetS in patients with RA [16,18,19]. Toms et al. [18] suggested that the anti-inflammatory effect of methotrexate and concurrent folic acid supplementation may contribute to the decreased frequency of MetS in patients with RA. However, these findings are not consistent with other studies [12,31]. Our study also showed no significant association between methotrexate use and MetS in patients with RA. A better understanding of the differences among these studies will require further investigation to elucidate a plausible protective effect of methotrexate against MetS in patients with RA.
In addition, the use of GCs did not significantly contribute to the presence of MetS in patients with RA in previous reports [14,15,19]. As the majority of patients in our study were taking GCs, we assessed the OR of cumulative GC dose instead of GC use, and no significant relationship between GCs and MetS in patients with RA was observed, similar to previous reports. It has been recognized that GCs have a deleterious effect on blood pressure, insulin resistance, and lipid metabolism [32] and that GCs lead to increase CVD in patients with RA [33]. GCs also have anti-inflammatory and immunosuppressive properties that could counteract undesirable side effects in patients with RA [34]. Hence, verifying the contribution of GCs to MetS in patients with RA is complicated by the intricacy of GC actions.
Among various direct and indirect methods for measuring insulin resistance, HOMA-IR, derived from fasting blood insulin and glucose concentrations, is a simple and useful clinical index, particularly in epidemiological studies [8,35]. In our study, both serum CRP and DAS28-ESR were independent risk factors for increased HOMA-IR in female patients with RA. These findings largely agreed with some previous studies evaluating predictors for HOMA-IR in patients with RA [36-38]. Over the last decade, increasing evidence has suggested a relationship between chronic inflammation and insulin resistance in RA [39]. Proinflammatory cytokines such as TNF-α and IL-6 are key players in the pathogenesis of RA and are closely related to insulin resistance [6,7,39]. Furthermore, the role of various adipokines in both RA and insulin resistance has been highlighted recently [6]. Taken together, our results suggest a connection between inflammation and insulin resistance in patients with RA.
In the present study, HOMA-IR was significantly higher in female patients with RA than in healthy subjects, which agreed with previous studies [14,24,36,38]. Taken together, these findings suggest that patients with RA had significantly increased insulin resistance compared with healthy controls. However, considering that insulin resistance is the major catalyst in MetS, the lack of a significant difference in the frequency of MetS between patients with RA and healthy controls in our study was unforeseen and interesting. Similarly, Karimi et al. [24] also demonstrated higher HOMA-IR in women patients with RA compared with controls, yet there was no difference in the prevalence of MetS between the two groups. We assumed that the complexity in MetS characteristics could explain these findings. MetS is a constellation of different, but correlated, metabolic abnormalities rather than a particular disease entity, and insulin resistance may be necessary, but not sufficient, for MetS [40,41]. Hence, increased HOMA-IR may not always result in the presence of MetS.
Whether patients with RA have significantly higher probabilities of traditional cardiovascular risk factors such as high blood pressure, dyslipidemia, diabetes, and smoking compared with those in the general population is somewhat controversial [30]. In our study, diastolic blood pressure and the frequency of the NCEP-ATP III 2004 criteria for high blood pressure were significantly higher in patients with RA than in healthy controls (Tables 1 and 2). Numerous factors, including obesity, inflammation, physical inactivity, and medications, may increase blood pressure in patients with RA [42]. Therefore, our findings are unlikely to be generalizable. In addition, HDL-C was significantly higher in patients with RA than in healthy subjects (Table 1). High disease activity in patients with RA is associated with low HDL-C levels, and antirheumatic treatment, including GCs, can reverse this dyslipidemia [43]. As mentioned above, most patients with RA in our study were taking antirheumatic medications that can alleviate dyslipidemia.
The findings in the present study must be considered in light of major limitations. First, this was an observational study with a cross-sectional design. The majority of our patients with RA were receiving GCs and DMARDs. Thus, we could not fully adjust for the effect of various medications on MetS and insulin resistance. Second, subjects in our study were recruited from only a single center located in a harbor city. Hence, most of our study subjects resided in a seacoast region, which was assumed to affect the overall frequency of MetS in our study (RA, 19.6%; healthy controls, 15.6%). Similarly, the prevalence of MetS in 2,519 healthy female subjects in the same center, reported by Kang et al. [44] in 2008, was 15.6% according to criteria used in our study. However, using the same criteria, the prevalence of MetS in a nationwide survey in South Korea in 2005 was 38.7% [45], which appears to be higher than that in our center. Taken together, we speculate that the geographic characteristics of the area could have acted as a confounding factor that affected the difference in MetS frequency between patients with RA and controls. Therefore, our results should be carefully interpreted. Last, the number of subjects in the present study might not have been sufficient to investigate all potential associated factors.
In conclusion, the frequency of MetS in South Korean women with RA was comparable to that in healthy subjects, although HOMA-IR was significantly higher in patients with RA than in healthy subjects. As many epidemiological factors, particularly ethnicity, may affect the frequency of MetS in patients with RA, it is still not clear whether patients with RA have a higher prevalence of MetS. Further studies are needed to confirm the relationship between RA and MetS.
KEY MESSAGE
1. The frequency of metabolic syndrome in South Korean women with rheumatoid arthritis (RA) was similar to than in that in healthy subjects.
2. RA patients had a higher homeostatic model assessment of insulin resistance (HOMA-IR), a measure of insulin resistance, than healthy subjects.
3. In RA patients, higher C-reactive protein and disease activity were associated with increased HOMA-IR.
Acknowledgments
We thank the late professor Sung-Il Kim who devoted himself to patient care, research, and education at the Division of Rheumatology, Department of Internal Medicine, Pusan National University School of Medicine.
Notes
No potential conflict of interest relevant to this article is reported.