Langerhans Cell Histiocytosis Followed by Hodgkin's Lymphoma
Article information
Abstract
A 22-year-old man was referred to our institution due to lower back pain and was diagnosed with Langerhans cell histiocytosis of the thoracic and lumbar spine. The patient achieved complete remission with radiotherapy and chemotherapy. One year later, right cervical lymphadenopathy was observed and Hodgkin's lymphoma was confirmed on biopsy. The patient was treated with chemotherapy and autologous stem cell transplantation, and experienced no further symptoms. Further, no evidence of recurrence was observed on follow-up imaging. This report discusses the association between Langerhans cell histiocytosis and Hodgkin's lymphoma.
INTRODUCTION
Langerhans cell histiocytosis (LCH) is a disease characterized by the proliferation of Langerhans cells, which are dendritic cells that express CD1a and S100 and contain Birbeck granules [1]. LCH has a complex relationship with malignant neoplasms; however, the association is rare and has been the subject of isolated case reports. The literature contains several reports of cases in which malignant lymphoma follows LCH, but fewer cases in which LCH follows malignant lymphoma [1-5]. Here, we describe a case of LCH followed by Hodgkin's lymphoma (HL) and discuss the association between LCH and HL.
CASE REPORT
A 22-year-old man with lower back pain of one month in duration was referred to our institution. The results of laboratory investigations and neurological examinations were normal. A bone scan, computed tomography (CT) scan and magnetic resonance imaging (MRI) suggested tuberculous spondylitis on the thoracic and lumbar spine. The patient underwent anterior thoracic corpectomy to obtain specimens for pathologic examination. No acid-fast bacilli were identified by Ziehl-Neelsen staining. An excisional biopsy of the osteolytic T2 spinal lesion showed extensive intramedullary proliferation of histiocytic cells with folded nuclei, numerous eosinophils, phagocytic histiocytes, and fibroblasts (Fig. 1). The bone spicules were thinned with numerous osteoblasts and osteoclasts. Immunostaining assays for CD1a and S100 were strongly positive in histiocytes with folded nuclei, suggesting LCH. Whole body positron emission tomography (PET; 18F-fluorodeoxyglucose, 18F-FDG) revealed other lesions in the sternum and left sacral ala. The multiple lesions of the spine, sternum, and sacrum were treated with involved-field radiotherapy. Subsequent FDG-PET scans revealed that the original lesions had completely disappeared, but that new hypermetabolic lesions had appeared in the subcarinal lymph node, left hilar lymph node, and left ischium. We determined that LCH had become systemic and the patient was treated with a combination of various chemotherapeutic agents in six cycles of cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP). Complete remission was achieved.
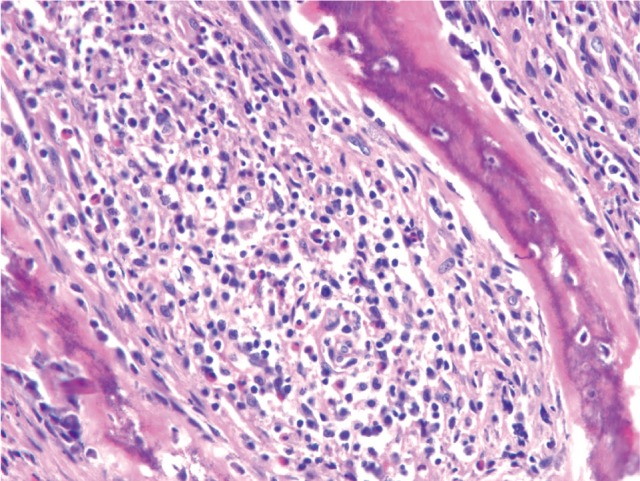
Biopsy specimen of the T2 spinal lesion showed intramedullary proliferation of histiocytic cells with folded nuclei, numerous eosinophils, and phagocytic histiocytes, accompanied by dense collagenous fibrosis and vascular proliferation (H&E, × 100).
One year after the patient entered remission, he developed right cervical lymphadenopathy. No other symptoms were reported. An excisional biopsy of the right cervical lymph node was performed. The extracted lymph node was smooth and measured 4.6 × 3.7 × 1.5 cm (Fig. 2). The cut surface was a glistening tan yellow color and displayed diffuse nodularity.
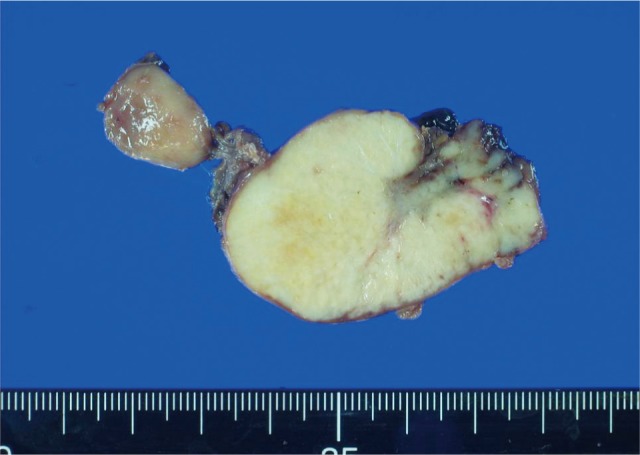
The cut surface of the enlarged lymph node was a glistening yellowish-tan color with diffuse nodularity.
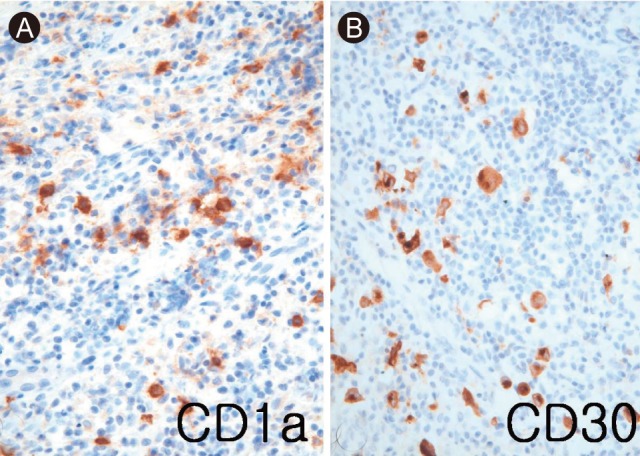
Microscopic examination at a magnification of × 100 showed a broad sclerotic fibrous band dividing the lymph node into multiple nodules containing large, scattered, and atypical cells (Fig. 3A). Further examination at a higher magnification showed that the atypical cells were either mononucleated or binucleated, with prominent nucleoli and abundant cytoplasm (Fig. 3B). Residual LCH was observed within fibrous bands around the nodules. Immunostaining revealed CD1a-positive Langerhans cells in the residual histiocytes within the sclerotic bands, and large, atypical, CD30 (Ki-1)-positive cells (× 400). We diagnosed the patient with HL secondary to LCH (Fig. 4). FDG-PET imaging and CT scans of the patient's thorax, abdomen, and pelvis revealed involvement of the right lung, right level II cervical lymph nodes, T12, L5, right 3rd rib anterior arc, right ischium, and right ilium. The patient received three cycles of ESHAP (methylprednisolone, etoposide, cisplatin, and cytosine arabinoside) chemotherapy, followed by high-dose chemotherapy (busulfan, etoposide, and cyclophosphamide) and autologous stem cell transplantation (ASCT). Ten months after ASCT, the patient reported no further symptoms of the disease, and FDG-PET scans were negative for hypermetabolic lesions.

(A) Microscopic examination showed broad sclerotic fibrous bands dividing the lymph node into multiple nodules (H&E, × 100). (B) The lymph node showed residual histiocytosis in the left field and classical Hodgkin's lymphoma in the right field (H&E, × 400).
DISCUSSION
Langerhans cells are non-pigmented, bone marrow-derived dendritic cells. As such, they function as antigen-presenting cells that are vital to the epidermal component of the immune system [3,6]. Langerhans cells share certain properties with monocytes and macrophages, but differ from other histiocytes in that they are CD1a-positive, with pale clefted nuclei, abundant pale eosinophilic cytoplasm, and Birbeck granules [1]. Their most striking histological feature, which is absent from HL, is the presence of eosinophils. In contrast, HL has histologically distinct Reed-Sternberg cells that are not present in LCH [6].
LCH manifests clinically in various ways, ranging from solitary bone lesions to polysystemic disease. The condition is occasionally fatal and is classified as a dendritic disorder by the World Health Organization classification of 1997 [7,8]. LCH resembles a malignant disease due to its progressive and/or invasive growth and dissemination. Depending on the clinical course and classification, LCH can be treated with many different therapeutic strategies, including benign neglect and observation, local treatment, immunomodulation, irradiation, chemotherapy, and allogenic stem cell transplantation [7]. For progressive multisystemic disease, various combinations of three- or four-agent chemotherapy regimens are used to treat higher risk lymphoma. Stem cell transplantation also benefits patients with bone marrow involvement [9,10].
The association between HL and LCH remains unclear. Until recently, coincidental LCH and HL were reported more often than could be expected on a case-by-case basis. In particular, most lymphomas associated with LCH were of the classic HL type. Egeler et al. [3] studied 39 patients with LCH and malignant neoplasia, and found an association between the two conditions. Twenty-five of the patients had both LCH and HL. That report suggested that LCH is associated with lymphoma, wherein the timing and unifocal involvement suggest a reactive process. Burns et al. [4] speculated that malignant lymphoma may directly or indirectly stimulate the proliferation of LCH. Adu-Poku et al. [1] presented a case of sequential discordant malignant lymphoma and suggested a degree of interdependence between the development of LCH and malignant lymphoma. In the present case, we found no histological evidence to explain the coincidental LCH and HL. However, many reports of an association between LCH and HL exist in the literature. In some cases, histological examination revealed lymphoid cells and Langerhans cells in the same lymph node. Furthermore, the year-long interval between the development of LCH and HL suggests an association between the two diseases that is unrelated to chemotherapy or radiation treatments. Such an association may involve a malignant change or modulation of the monocyte-histiocyte system. For example, Langerhans cells may themselves be premalignant, or may provide an immunologic environment conducive to the development, growth, and promotion of malignant cells.
In conclusion, we describe a case in which LCH developed after HL. The significance of this association should be further explored in future studies, especially in regard to the clinical aspects of the disease course and management. In addition, the mechanism underlying the association between LCH and HL must be elucidated.
Notes
No potential conflict of interest relevant to this article is reported.