Effects of Add-On Therapy with NDC-052, an Extract from Magnoliae Flos, in Adult Asthmatic Patients Receiving Inhaled Corticosteroids
Article information
Abstract
Background/Aims
There is a need for new anti-asthmatic medications with fewer side effects. NDC-052, an extract of the medicinal herb Magnoliae flos, which has a long history of clinical use, was recently found to have anti-inflammatory effects. Herein, we evaluated the effects of NDC-052 as an add-on therapy in patients with mild to moderate asthma using inhaled corticosteroids (ICS).
Methods
In a non-comparative, multi-center trial, 148 patients taking ICS received NDC-052 for eight weeks. We evaluated their forced expiratory volume in one second (FEV1), morning and evening peak expiratory flow rate (AM and PM PEFR), AM/PM asthma symptom scores, visual analogue symptom (VAS) scores, night-time wakening, frequency of short-acting β2-agonist usage, and adverse events.
Results
After eight weeks, both AM and PM PEFRs were significantly improved. Asthma symptom scores, VAS scores, the frequency of nights without awakening, and the frequency of β2-agonist use were also reduced. Most of the adverse drug reactions were mild and resolved spontaneously.
Conclusions
The addition of NDC-052 to ICS had a beneficial effect on asthma control in patients with mild to moderate asthma, with good tolerability and fewer side effects. Further studies are necessary to evaluate the effects of NDC-052 in patients with severe and/or refractory asthma.
INTRODUCTION
Asthma is a common chronic inflammatory disease of the airways that is responsible for significant morbidity, mortality, and healthcare costs [1,2]. Airway inflammation is a key pathophysiologic feature of asthma, leading to airway obstruction, airway hyperresponsiveness (AHR), and even remodeling of the airway. Recent guidelines for asthma management have therefore emphasized a need for the control of airway inflammation using inhaled corticosteroids (ICS) [3,4]. Chronic use of high-dose ICS, however, may result in serious side effects, including adrenal suppression and reduction in growth velocity, especially in children [5-7]. In addition, adequate ICS and other controller medications cannot achieve asthma control in some patients. Consequently, there is a pressing need for new alternative and/or complementary drugs with fewer side effects.
Recently, a number of clinical and laboratory studies have investigated the anti-asthmatic effects of Chinese herbal products. Some of these herbal remedies have been shown to be effective and safe alternatives or complements to standard therapy for asthma and other allergic diseases [8]. Magnoliae flos (Chinese name, 辛夷, Xin-yi) has a long history of clinical use in Asian countries, and is used to treat ulceration of the nasal mucosa, allergic rhinitis, sinusitis, bronchial asthma, and other conditions [9]. NDC-052 (Han Kook Sin Yak Pharmaceutical Co. Ltd., Nonsan, Korea) is a purified extract of M. fargesii and was developed to enhance and control bioactive lignan content, including magnolin and epimagnolin A [10]. These bioactive lignans have been shown to inhibit various inflammatory mediators, such as platelet-activating factor (PAF), cytosolic phospholipase A2, hexosaminidase, lipoxygenase, the complement system, and production of leukotriene C4 [11,12]. A recent study demonstrated that epimagnolin decreased nitric oxide (NO) levels by modulating inducible nitric oxide synthase (iNOS) in human respiratory epithelial cells [13]. In addition, oral administration of 50 mg/kg NDC-052 for eight weeks reduced airway resistance in a guinea pig chronic asthma model (unpublished data). Taken together, these reports suggest that concentrated NDC-052 extract might have anti-inflammatory, as well as anti-asthmatic effects.
Prior to the present study, the Korea Food & Drug Administration approved NDC-052 for clinical trials, and a phase II pilot study of NDC-052 in patients with persistent mild asthma found that a dose of 600 mg/day was effective and safe. We have evaluated the effects of NDC-052 as an add-on therapy to ICS in patients with persistent mild to moderate asthma symptoms.
METHODS
Study design
This prospective, multi-center, non-comparative study was conducted in six university hospitals in Korea. After screening (visit 1), patients who voluntarily agreed to participate underwent a two-week 'run-in' period, during which they discontinued all asthma medications except ICS, which they had taken for at least four weeks. At the end of the run-in period (visit 2), we selected suitable subjects based on pulmonary function tests, symptom diaries, and laboratory data. At this point, 'well-controlled' asthma patients were excluded. 'Well-controlled' asthma was defined as an average of < 2 points on the asthma symptom score test, calculated as the sum of the following items (one point per item): no night-time awakening caused by asthma, no exacerbation of asthma symptoms, no usage of rescue medications, less than 20% peak expiratory flow rate (PEFR) variation, and no change in medications for the control of asthma symptoms, except for medications prescribed for one week. Patients who met the inclusion criteria were given NDC-052 (600 mg/day) for eight weeks. At subsequent four-week intervals (visits 3 and 4), we evaluated their pulmonary function, clinical and laboratory parameters, and adverse reactions.
This study was conducted in accordance with the Declaration of Helsinki and in compliance with Korean Good Clinical Practice and International Conference on Harmonization (ICH) Good Clinical Practice Guidelines. Each patient provided written informed consent before initiation of any study procedures. The study protocol and a copy of the informed consent document were reviewed and approved by the Institutional Review Board of each study center.
Subjects
We included study subjects aged 18-70 years with an at least three month history of asthma symptoms. Inclusion criteria at visit 1 included: 1) FEV1 ≥ 60% of the predicted value; 2) FEV1 increased ≥ 15% after the administration of either short-acting β2-agonist or oral corticosteroid (20-40 mg/day) for 2-3 weeks; 3) FEV1 decreased ≥ 20% in response to the methacholine bronchial provocation test, with a PC20 value of less than 25 mg/mL; and 4) mild to moderate persistent asthma symptoms, based on the 2006 Global Initiative for Asthma (GINA) diagnostic criteria. Patients were excluded if they had any other pulmonary diseases, any severe co-morbidity, or had smoked more than 10 packs/yr and stopped smoking four weeks before screening. We also excluded women who were pregnant or breast-feeding.
Pulmonary function tests and clinical evaluations
FEV1 was measured at every visit and daily AM/PM PEFR was measured with a mini peak flow meter. The PEF was measured in the standing position immediately after waking in the morning and before going to bed in the evening. Patients were asked to measure PEF before taking medications, if possible, and were not asked to discontinue medications, including controllers, during the monitoring period. On each occasion, the best of at least three attempts was recorded. PEFR variability was defined by the following equation and was averaged over the period between each visit: (high PEF - low PEF) / mean PEF of the day × 100. We asked patients to assess symptom severity for two weeks before each visit, using a 100 mm visual analogue scale (VAS), ranging from no symptoms (0 mm) to the worst ever symptom (100 mm). This scale was used as a responsive outcome measurement and had been validated in previous clinical studies of patients with bronchial asthma [14,15]. Daytime asthma symptoms were scored as none (0), once for a short period of time (1), twice or more for short periods of time (2), all day without any major impact on normal daily activities (3), all day with an impact on normal daily activities (4), and symptoms so severe that it was impossible to conduct normal daily activities or work (5). Nocturnal asthma symptoms were scored as none (0), one awakening due to symptoms (1), two or more awakenings due to symptoms (2), and could not sleep at all because of symptoms (3). The frequency of nocturnal awakenings was defined as the frequency of days without night awakening due to asthma symptoms. The frequency of use for rescue medications was measured as the number of days that patients used these medications.
Safety evaluation
Safety data were obtained over eight weeks of treatment in all subjects. Causality of the event was assessed by the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) criteria [16]. We graded severity as mild, moderate, or severe. Laboratory test results at each visit were compared with data obtained at the time of screening.
Statistical analysis
Results are shown as mean ± SD. The mean daytime and nocturnal asthma symptom scores were analyzed for the entire period between visits. All data on '0 week' were obtained from a two-week 'run-in' period. Paired t tests were used to evaluate changes between baseline and after eight weeks of administration of the study drug. A p value < 0.05 was considered statistically significant. All statistical tests were performed using SAS version 8.0 (SAS Institute, Cary, NC, USA). For safety analysis, all abnormal events were placed into tables and their frequencies were calculated.
RESULTS
Subject characteristics
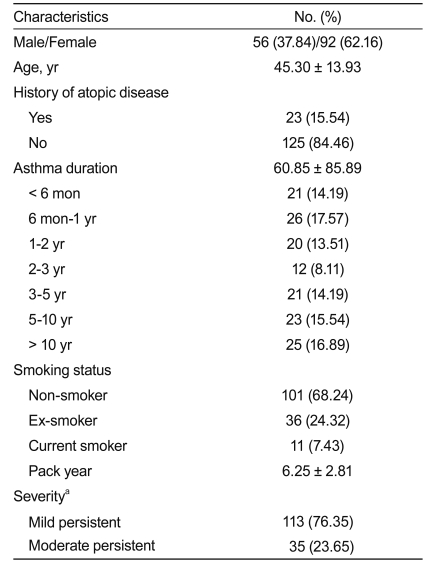
Of the 179 patients screened, 148 were enrolled and received NDC-052. These 148 patients included 56 (37.84%) males and 92 (62.16%) females. Based on the GINA guidelines for severity, 113 subjects had mild persistent and 35 had moderate persistent asthma. Of the enrolled subjects, 101 (68.24%) were non-smokers, 11 (7.43%) were current smokers, and 36 (24.32%) were exsmokers (Table 1).
Effects of add-on ND C-052 on pulmonary function
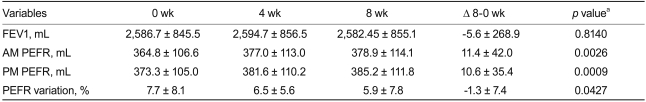
Treatment with add-on NDC-052 for eight weeks resulted in increases in mean AM and PM PEFR from baseline, and a decrease in mean daily PEFR variation. However, NDC-052 treatment did not result in a statistically significant improvement in FEV1 (Table 2).
Effects of add-on ND C-052 on clinical parameters
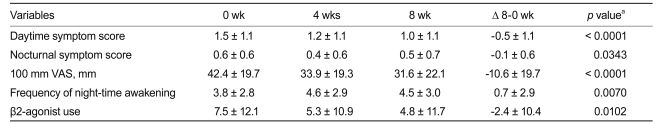
We found that eight weeks of add-on NDC-25 resulted in significant improvements in both daytime and nocturnal symptom scores. In addition, add-on treatment reduced 100 mm VAS scores, as well as reduced the frequency of nighttime awakening and use of rescue medication after eight weeks (Table 3).
Safety evaluation
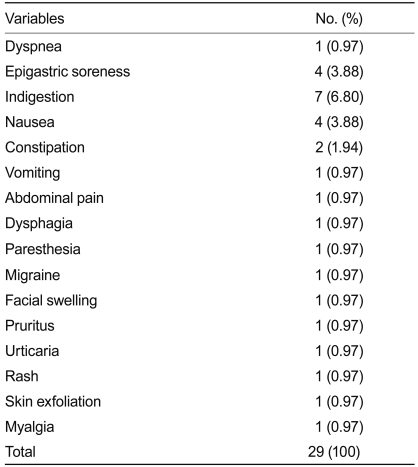
A total of 103 adverse events were reported by 52 patients. Analysis of the causality between adverse events and NDC-052 showed that only one event (0.97%) was 'probable,' 28 events (27.18%) were 'possible,' and 74 (71.84%) were 'unlikely.' The 29 adverse events classified as 'probably' and 'possibly' associated with NDC-052 are shown in Table 4. Although five events (4.85%) were classified as severe, none were correlated with NDC-052. Laboratory findings at screening and eight weeks showed no significantly abnormal findings.
DISCUSSION
We have shown here that the addition of NDC-052, an extract of an herbal remedy, to ICS may play a role in improving asthma control in patients with mild to moderate asthma. NDC-052 improved the results of pulmonary function tests, such as AM and PM PEFR and PEFR variation, as well as clinical parameters, including asthma symptom VAS, daytime and nocturnal asthma symptoms, the frequency of night-time awakening, and the frequency of β2-agonist use. In addition, NDC-052 was well tolerated and no severe adverse reactions were observed.
Traditional Chinese medicine (TCM) is one of the oldest types of medical practice in the world, and has played an important role for centuries in preventing and treating diseases in China and other Asian countries. Recently, TCM has been tested for the treatment of allergic diseases, including asthma, allergic rhinitis, and food allergy. The anti-asthma herbal medicines being evaluated include MSSM-002, modified Mai Men Dong Tang, Ding Chuan Tang, and STA-1, with clinical trials suggesting that these TCM remedies are efficacious and safe alternatives or complements to standard Western asthma therapy [8,17,18].
In this context, NDC-052 extracted from M. flos using ethanol, is a well-known natural herbal remedy in East Asian countries, including Korea, China, and Japan. M. flos refers to the flower buds of M. denudata and other plants belonging to the same genus, including M. fargesii (also known as M. biondii), M. denudata, M. liliflora, M. salicifolia, and M. kobus. Extraction yields many compounds, including oils, lignan compounds, and sesquiterpenes [10,19,20]. Some of the compounds extracted from both M. fargesii and M. denudata were shown to be calcium antagonists, inhibiting the activity of PAF and serum complement, and reducing tumor necrosis factor-alpha (TNF-alpha) production [11,12,20-23]. Using an anaphylaxis model in mice, M. flos was shown to inhibit immediate-type allergic reactions by inhibiting mast cell degranulation, both in vivo and in vitro [24]. Moreover, epimagnolin, a major lignin of NDC-052, was shown to inhibit the expression of iNOS and the production of NO via extracellular signal-regulated kinase (ERK) pathway in cytokine-stimulated human respiratory epithelial cells [13]. Thus, NDC-052 may have both anti-inflammatory and antioxidant effects, and the pathway by which NDC-052 reduces inflammation may differ from that of steroids. Therefore, NDC-052 may provide additional benefits to asthma patients using ICS.
We found that treatment with add-on NDC-052 did not noticeably increase FEV1. Most of our subjects, however, had mild persistent asthma, with FEV1 > 80% of the predicted value. Nevertheless, NDC-052 improved AM and PM PEFR and other clinical indicators of asthma control, such as the need for β-agonist relief therapy and both day and night asthma symptom scores. In recent guidelines, these clinical outcomes were regarded as important characteristics for evaluating asthma control. Therefore, our data indicate that patients with close-tonormal FEV1 levels may be symptomatic and require additional control of asthma symptoms. In addition, our results were similar to those of previous studies showing that addition of ICS to leukotriene receptor antagonists, currently used as alternatives to ICS in patients with mild persistent asthma, improved various measurements of asthma control, despite failing to cause a significant improvement in FEV1 [25,26]. Pharmacologically, NDC-052 has anti-inflammatory, rather than bronchodilating, effects. Therefore, NDC-052 may not directly affect FEV1 level. We did not measure inflammatory markers in these patients, suggesting the need for additional clinical studies involving measurements of inflammatory markers, such as induced sputum analysis or exhaled NO, to determine the mechanisms by which NDC-052 affects airway inflammation. Further clinical trials are necessary to determine if the addition of NDC-052 to ICS is effective in patients with more severe or refractory asthma, or if it can decrease the frequency of asthma exacerbation.
ICS has been shown to improve both asthma-related symptoms and pulmonary function in many patients, although a subset of patients required additional medications to achieve asthma control. Updated GINA guidelines suggest that symptoms can be improved by: 1) increasing the dose of ICS, 2) adding inhaled long-acting β2-agonists (LABA), or 3) adding other controller medications, such as leukotriene modifiers and sustained-release theophylline. Since ICS has a plateau effect, such that increased doses are not matched by further increases in efficacy [27,28], the updated GINA recommends the addition of a controller, preferably LABA, rather than increasing the dose of ICS. LABA, however, does not have anti-inflammatory effects, raising concerns that it may enhance airway remodeling [29]. In addition, inhaled agents may fail to reach the small airways, i.e., the site of inflammation in asthma. Theophylline, a currently available oral controller, has a narrow therapeutic window and many side effects, limiting its utility as an add-on therapy in asthmatics suboptimally controlled with ICS. These findings indicate the need for novel alternative agents that can improve airway inflammation and asthma symptoms in patients who cannot be controlled by ICS alone.
However, the design of this study imposes some limitations on the interpretation of the results. First, the absence of a placebo group precluded assessment of any placebo effect on asthma symptoms; thus, improvements in outcome measures may have been influenced by the expectation of a benefit from treatment. Secondly, the increased compliance to ICS may have affected the results. However, we strongly suggested that participants use their ICS regularly during the whole study period, including run-in periods, and enrolled symptomatic patients despite regular ICS usage. Finally, eight weeks was a relatively short period to evaluate the long-term effects of therapeutic drugs on asthma control. Further clinical trials are necessary to address these issues.
In conclusion, we have shown that add-on NDC-052 was well tolerated and shows potential for improving asthma control in patients with persistent symptoms treated with ICS. Further clinical trials are needed to test the efficacy and safety of NDC-052 in patients with severe and/or refractory asthma.
Notes
This study was financially supported by Han Kook Sin Yak Pharmaceutical Co. Ltd., Nonsan, Korea.