Role of IL-1α in Cisplatin-Induced Acute Renal Failure in Mice
Article information
Abstract
Background/Aims
For unknown reasons, caspase-1 -/- mice, protected against cisplatin-induced acute renal failure (ARF), are deficient in interleukin (IL)-1α. We thus asked whether IL-1α deficiency underlies the mechanism of protection against cisplatin-induced ARF in these mice.
Methods
Cisplatin (30 mg/kg) was injected intraperitoneally into wild-type C57BL/6 mice to produce a cisplatin-induced model of ARF. IL-1α was measured in control vehicle- and cisplatin-treated wild-type animals. We also examined whether IL-1α -/- mice were similarly protected against cisplatin-induced ARF. Additionally, infiltration of CD11b- and CD49b-positive cells, as markers of macrophages, natural killer, and natural killer T cells (pan-NK cells), was investigated in wild-type and IL-1α -/- mice.
Results
Compared with vehicle-treated mice, renal IL-1α increased in cisplatin-treated wild-type mice beginning on day 1. IL-1α -/- mice were shown to be protected against cisplatin-induced ARF. No significant difference in the infiltration of neutrophils or CD11b- and CD49b-positive cells were observed between wild-type and IL-1α -/- mice.
Conclusions
Mice deficient in IL-1α are protected against cisplatin-induced ARF. The lack of IL-1α may explain, at least in part, the protection against cisplatin-induced ARF observed in caspase-1 -/- mice. Investigation of the protective mechanism (s) in IL-1α -/- mice in cisplatin-induced ARF merits further study.
INTRODUCTION
The potent antineoplastic agent cisplatin is highly nephrotoxic and is known to cause acute renal failure (ARF), which limits its use as a chemotherapeutic agent [1]. As one of the most widely studied nephrotoxins, the pathogenesis of cisplatin-induced ARF has been the focus of several studies, with the goal of developing adjunctive therapies.
In vitro, cisplatin causes apoptosis or necrosis of renal proximal tubular cells, depending on the dose [2]. In vivo, histological studies have shown apoptosis and necrosis as well as inflammation of renal tissue [3-7]. Proinflammatory effects in cisplatin-induced ARF have been well-documented and include increases in the mRNA levels of proinflammatory cytokines, adhesion molecules, and chemokines [3,8]. Accordingly, anti-inflammatory strategies have been developed to inhibit cisplatin-induced ARF, such as anti-TNF-α [3,4], interleukin (IL)-10 [5], and anti-intercellular adhesion molecule-1 (ICAM-1) [6]. Faubel et al. [9] demonstrated that renal caspase-1 activity was increased in cisplatin-induced ARF and that renal dysfunction, tubular necrosis, and neutrophil infiltration were reduced in caspase-1 -/- mice. Caspase-1 converts the precursor forms of the proinflammatory cytokines IL-1β and IL-18 to their mature forms [10,11]. It was thus suggested that caspase-1 -/- mice are protected against cisplatin-induced ARF because of a reduction in these proinflammatory cytokines and in renal neutrophil infiltration [9]. However, prevention of the increases in renal IL-1β, IL-18, and neutrophils is insufficient to inhibit cisplatin-induced ARF [10].
Although IL-1α is not a known substrate of caspase-1, it has been observed that caspase-1 -/- mice are also deficient in IL-1α [11]. Because the findings regarding IL-1β, IL-18, and neutrophil inhibition were negative, we asked whether the IL-1α deficiency could explain the protection of caspase-1 -/- mice against cisplatin-induced ARF and thus whether this cytokine played a role in the pathogenesis of the disease.
METHODS
Animals and drug administration
IL-1α -/- mice were provided by Dr. Yichiro Iwakura of the Institute of Medical Science at the University of Tokyo, Japan. IL-1α deficiency was confirmed by enhanced chemiluminescence for IL-1α in the spleen and kidney (Fig. 1). Age-matched C57BL/6 mice (Harlan, Indianapolis, IN and Jackson Labs, Bar Harbor, ME, USA) weighing 20-25 g were used as controls for IL-1α -/- mice.
Cisplatin-induced ARF
Mice were maintained on a standard diet and water was freely available. Animals were housed five to a cage under a 12/12-hour light/dark schedule for at least 1 week before cisplatin treatment. Food and water were withheld for 12 hours prior to administration of the drug. Cisplatin was injected intraperitoneally at a dose of 30 mg/kg, after which the mice again had free access to food and water. The mice were anesthetized with Avertin (2, 2, 2-tribromoethanol; Aldrich, Milwaukee, WI, USA) and their kidneys then removed. Additionally, blood samples were collected via cardiac puncture 1, 2, or 3 days after cisplatin administration.
Drug preparation
Cisplatin (Aldrich) was prepared freshly on the day of administration in normal saline at a concentration of 1 mg/mL. Mice were given either 30 mg cisplatin/kg body weight or the same volume of vehicle (Veh; saline).
Blood urea nitrogen and serum creatinine measurements
Blood urea nitrogen (BUN) and serum creatinine were measured using a BUN and creatinine autoanalyzer (Beckman Instruments, Fullerton, CA, USA).
Enhanced chemiluminescence assay for IL-1α
The enhanced chemiluminescence assay, detecting both the precursor and mature forms of IL-1α, was performed using whole-kidney homogenates as described previously in detail [12].
Histological acute tubular necrosis (ATN), apoptosis scoring, and neutrophil infiltration
Kidneys fixed in 4% paraformaldehyde and paraffin-embedded were sectioned at 4 µm and stained with periodic acid-Schiff (PAS) by standard methods. All histological examinations were performed by a renal pathologist in a blinded fashion. Histological changes due to ATN were evaluated in the outer strip of the outer medulla on PAS-stained tissue and quantified by counting the proportion of tubules that displayed cell necrosis, brush-border loss, cast formation, and tubule dilation, as follows: 0, none; 1, < 10%; 2, 11-25%; 3, 26-45%; 4, 46-75%; and 5, > 76%. At least 10 fields (× 200) were reviewed on each slide. Morphological criteria, including cellular rounding and shrinkage, nuclear chromatin compaction, and the formation of apoptotic bodies, were used to determine apoptotic cells on PAS-stained tissue. Cells were counted by a pathologist experienced in the evaluation of renal apoptosis. Apoptotic tubular cells were quantitatively assessed per high-powered field in the outer strip of the outer medulla by a renal pathologist in a blinded fashion. At least 10 fields were counted on each slide. Neutrophil infiltration was assessed quantitatively on PAS-stained tissue by a renal pathologist by counting the number of neutrophils per high-powered field (× 400). At least 10 fields comprising the outer strip of the outer medulla were counted on each slide.
Immunofluorescence for CD11b-positive cells as a marker of macrophages and CD49b-positive cells as a marker of pan-NK cells
Kidney tissues were embedded in optimal cutting temperature medium, snap-frozen in liquid nitrogen, and stored at -80℃ until sectioning. Cryostat sections (5 µm) were fixed in 70% acetone/30% methanol and prepared for immunofluorescence studies as described previously [13]. The primary antibodies used were rat anti-mouse CD11b monoclonal antibody (catalog no. MCA74; Serotec, Oxford, UK), as a macrophage marker, and purified anti-mouse CD49b (pan-NK cells; BioLegend, San Diego, CA, USA), for the determination of pan-NK cell infiltration. Fifteen randomly chosen high-powered fields (× 400) of the corticomedullary junction were assessed by a renal pathologist in a blinded fashion.
Statistical analyses
Values are expressed as the mean ± SE. Non-normally distributed data were analyzed by the non-parametric unpaired Mann-Whitney test. Multiple-group comparisons were assessed by the Kruskal-Wallis test using the post hoc Newman-Keuls test. p values < 0.05 were considered to indicate statistical significance.
RESULTS
Renal function in cisplatin-induced ARF
Renal dysfunction was most severe on day 3 whereas on days 1 and day 2 serum creatinine (Cr) and BUN were unchanged after cisplatin injection. On day 1, serum BUN and Cr (mg/dL) were 26.6 ± 3.3 and 0.5 ± 0.1 in the cisplatin-treated (Cis) group (p > 0.05 vs. Veh; BUN/Cr: 22.6 ± 3.0 and 0.3 ± 0.1; n = 15). On day 2, serum BUN and Cr were 46.2 ± 17.7 and 0.4 ± 0.2 in the Cis group (p > 0.05 vs. Veh, day 1; n = 15). On day 3, serum BUN and Cr were 222.0 ± 82.9 and 3.3 ± 1.7 in the Cis group (p < 0.001 vs. Veh, day 1, day 2; n = 15).
Renal IL-1α in cisplatin-induced ARF
Renal IL-1α was determined on days 1, 2, and 3 in vehicle- and cisplatin-treated wild-type mice. Compared with the vehicle-treated wild-type control, renal IL-1α increased on all 3 days in cisplatin-treated wild-type mice (n = 6; Fig. 2).
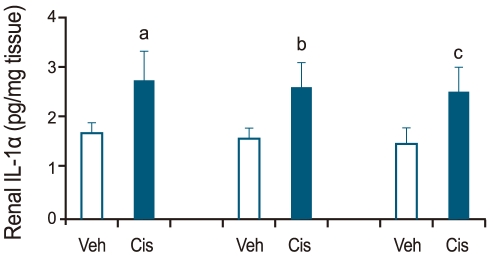
Renal interleukin (IL)-1α levels in cisplatin-induced acute renal failure. Renal IL-1α was determined on days 1, 2, and 3 in vehicle (Veh)- and cisplatin (Cis)-treated wild-type mice. Renal IL-1α was higher in cisplatin-treated than in vehicle-treated wild-type mice on days 1, 2, and 3 (a,b,cp < 0.05 vs. vehicle-treated wild type mice, n = 6).
Renal function in cisplatin-treated IL-1α -/- mice
IL-1α -/- mice were studied to investigate the role of IL-1α in cisplatin-induced ARF. Fig. 3 shows that BUN and Cr levels increased significantly in cisplatin- vs. vehicle-treated wild-type and IL-1α -/- mice (178 ± 16, 2.2 ± 0.3 vs. 22 ± 3, 0.2 ± 0.1; 25 ± 3, 0.2 ± 0.1, p < 0.05, n = 15). Additionally, there was a significant difference in the BUN and Cr levels of cisplatin-treated wild-type and cisplatin-treated IL-1α -/- mice (178 ± 16 and 2.2 ± 0.3 vs. 102 ± 12 and 1.5 ± 0.4; p < 0.05, n = 15).
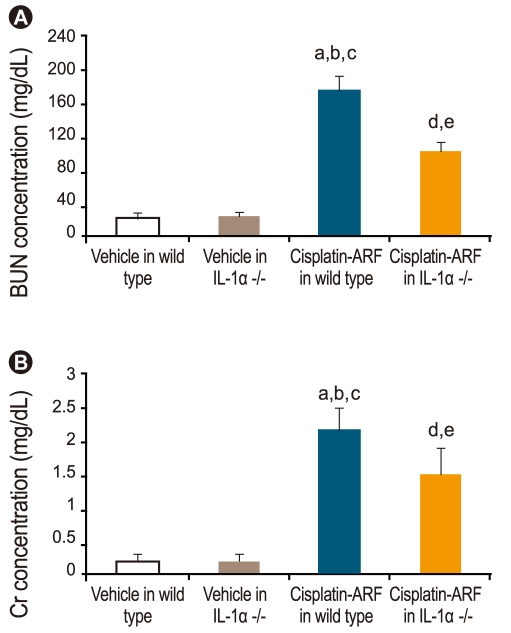
Renal function in cisplatin-treated interleukin (IL)-1α -/- mice. By day 3 of cisplatin injection, blood urea nitrogen (BUN) and creatinine level (mg/dL) had increased significantly in cisplatin-treated wild-type mice (a,b,cp < 0.05 vs. vehicle-treated wild-type and IL-1α -/- mice, cisplatin-treated IL-1α -/- mice, n = 15) and cisplatin-treated IL-1α -/- mice (d,ep < 0.05 vs. vehicle-treated wild-type and IL-1α -/- mice, n = 15). Also, there was a significant difference in BUN and creatinine level (mg/dL) between cisplatin-treated wild-type and cisplatin-treated IL-1α -/- mice (178 ± 16 and 2.2 ± 0.3 vs. 102 ± 12 and 1.5 ± 0.4; p < 0.05; n = 15). ARF, acute renal failure.
Acute tubular necrosis, apoptosis, and neutrophil score in cisplatin-treated IL-1α -/- mice
ATN scores increased significantly in cisplatin- vs. vehicle-treated wild type and IL-1α -/- mice (4.7 ± 0.2 vs. 0.3 ± 0.1, 0.2 ± 0.1, p < 0.05; n = 15; Fig. 4). A significant difference in the ATN scores of cisplatin-treated wild-type and IL-1α -/- mice was also noted (4.7 ± 0.2 vs. 3.3 ± 0.2; p < 0.05, n = 15). The number of apoptotic tubular cells per 10 high-powered fields was 0.2 ± 0.1 in vehicle-treated wild-type and IL-1α -/- mice, 13 ± 6 in cisplatin-treated wild-type mice, and 13 ± 4 in cisplatin-treated IL-1α -/- mice (n = 15). Neutrophil scores were significantly higher in cisplatin-treated wild-type mice than in vehicle-treated wild-type and IL-1α -/- mice (98.7 ± 15.1 vs. 1.3 ± 0.2 vs. 1.7 ± 0.2, p < 0.05; n = 15; Fig. 5). However, there was no significant difference in the neutrophil scores of cisplatin-treated wild-type and IL-1α -/- mice (98.7 ± 15.1 vs. 90.7 ± 12.5; p > 0.05; n = 15).

Acute tubular necrosis (ATN) scores in cisplatin-treated interleukin (IL)-1α -/- mice. By day 3 of cisplatin administration, ATN scores were increased significantly in cisplatin-treated wild-type (a,b,cp < 0.05 vs. vehicle-treated wild-type and IL-1α -/- mice, cisplatin-treated IL-1α -/- mice, n = 6) and cisplatin-treated IL-1α -/- mice (d,ep < 0.05 vs. vehicle-treated wild-type and IL-1α -/- mice, n = 6). Also, there was a significant difference in the ATN scores of cisplatin-treated wild-type vs. cisplatin-treated IL-1α -/- mice (4.7 ± 0.2 vs. 3.3 ± 0.2; p < 0.05; n = 15). ARF, acute renal failure.

Neutrophil score in cisplatin-treated interleukin (IL)-1α -/- mice. By day 3 of cisplatin administration, neutrophil scores had increased significantly in cisplatin-treated wild-type (a,bp < 0.05 vs. vehicle-treated wild-type and IL-1α -/- mice, n = 15) and cisplatin-treated IL-1α -/- mice (c,dp < 0.05 vs. vehicle-treated wild-type and IL-1α -/- mice, n = 15). However, there was no significant difference in the neutrophil scores of cisplatin-treated wild type and cisplatin-treated IL-1α -/- mice (98.7 ± 15.1 vs. 90.7 ± 12.5; p > 0.05, n = 15). ARF, acute renal failure.
Immunofluorescence staining for CD11b- and CD49-positive natural killer and natural killer T cells in cisplatin-treated IL-1α -/- mice
Infiltration of CD11b-positive cells was significantly higher in cisplatin-treated than in vehicle-treated wild-type and IL-1α -/- mice (10.2 ± 1.3; 10.6 ± 1.0; 1.2 ± 0.2; 1.4 ± 0.2, p < 0.05, n = 6; Fig. 6). However, there was no significant difference in the infiltration of CD11b-positive cells between cisplatin-treated wild-type and IL-1α -/- mice (10.2 ± 1.3; 10.6 ± 1.0; p > 0.05; n = 6). The infiltration of CD49b-positive cells was significantly higher in cisplatin-treated than in vehicle-treated wild-type and IL-1α -/- mice (5.7 ± 0.3; 5.2 ± 0.2; 0.4 ± 0.2; 0.7 ± 0.2, p < 0.05; n = 6; Fig. 7), but there was no significant difference in the infiltration of CD49b-positive cells between cisplatin-treated wild-type and IL-1α -/- mice (5.7 ± 0.3; 5.2 ± 0.2; p > 0.05; n = 6).

Immunofluorescence staining for CD11b (+) cells in cisplatin-treated interleukin (IL)-1α -/- mice. By day 3 of cisplatin administration, the infiltration of CD11b (+) cells had increased significantly in cisplatin-treated wild-type (a,bp < 0.05 vs. vehicle-treated wild-type and IL-1α -/- mice, n = 6) and IL-1α -/- mice (c,dp < 0.05 vs. vehicle-treated wild-type and IL-1α -/- mice, n = 6). However, there was no significant difference in the infiltration of CD11b (+) cells between cisplatin-treated wild-type and cisplatin-treated IL-1α -/- mice (10.2 ± 1.3; 10.6 ± 1.0; p > 0.05; n = 6). ARF, acute renal failure.

Immunofluorescence staining for CD49 (+) cells in cisplatin-treated interleukin (IL)-1α -/- mice. By 3 day of cisplatin administration, the infiltration of CD49b (+) cells had increased significantly in cisplatin-treated wild-type (a,bp < 0.05 vs. vehicle-treated wild-type and IL-1α -/- mice, n = 6) and IL-1α -/- mice (c,dp < 0.05 vs. vehicle-treated wild-type and IL-1α -/- mice, n = 6). However, there was no significant difference in the infiltration of CD49b (+) cells between cisplatin-treated wild-type and cisplatin-treated IL-1α -/- mice (5.7 ± 0.3; 5.2 ± 0.2; p > 0.05; n = 6). ARF, acute renal failure.
DISCUSSION
Recently, Faubel et al. [9] demonstrated that caspase-1 -/- mice failed to develop cisplatin-induced ARF. Caspase-1, formerly known as interleukin-1β converting enzyme, is a potent inflammatory agent because of its activation of IL-1β, a proximal mediator of the inflammatory events associated with infection, sepsis, and ischemia. However, a recent study found that although renal IL-1β increased prior to the onset of renal dysfunction and was reduced in caspase-1 -/- mice, inhibition of the IL-1 receptor did not protect against cisplatin-induced ARF. Thus, IL-1β does not appear to be an important mediator of cisplatin-induced ARF. These results are consistent with data from other models of ARF, including ischemic and endotoxemic ARF [14,15].
In addition to the activation of IL-1β, caspase-1 is known to activate the cytokine IL-18, formerly known as interferon-gamma-inducing factor. IL-18 is involved in a diverse array of functions related to inflammation [16]. However, while inhibition of IL-18 protected against ischemic ARF [17], IL-18 antiserum failed to prevent cisplatin-induced ARF. Moreover, transgenic mice overproducing IL-18 binding protein still developed the disease [10].
Activated neutrophils may up-regulate adhesion molecules, resulting in leukocyte adhesion and the obstruction of blood flow in the vasculature, further contributing to tubular injury [9]. Although renal neutrophils were not detected in mice treated with neutrophil-depleting antibody, neither renal function nor tubular necrosis improved in the mice with cisplatin-induced ARF. Thus, while neutrophil recruitment is associated with cisplatin-induced ARF, infiltrating neutrophils are not essential to the development of tubular necrosis and renal failure [10].
Caspase-1 -/- mice are also deficient in IL-1α [11]. Pro-IL-1α is cleaved to its active form by the calcium-dependent cysteine protease calpain. IL-1α lacks the aspartate cleavage sequence of caspase-1 and is thus not a substrate for the enzyme. Reasons for the reduced levels of IL-1α in caspase-1-deficient mice remain unknown. However, no protective effect in cisplatin-induced ARF was observed in response to inhibitors of IL-1β, IL-18, or neutrophils. Thus, in this study we examined whether IL-1α acted as a mediator of cisplatin-induced ARF.
Our results showed that cisplatin injection resulted in a significant increase in renal IL-1α levels 1 day later, thus preceding the increase in IL-1β and IL-18. IL-1α -/- mice were observed to have less functional and histological injury, as judged by serum Cr, BUN, and ATN scores. However, there was no difference in the number of apoptotic cells between IL-1α -/- and wild-type mice. Faubel et al. [9] determined that apoptosis was most severe on day 2 after cisplatin injection, while renal dysfunction was minimal, suggesting that apoptosis had only a small effect on renal dysfunction directly. Alternatively, serum Cr and BUN may be inappropriate markers to detect early changes in the glomerular filtration rate. Interestingly, the reduction in apoptosis on day 2 in caspase-1 -/- mice preceded the reduction in necrosis and renal dysfunction, both of which occurred on day 3 [9]. This time course of events suggests that apoptosis accounts for the necrosis and renal dysfunction seen on day 3 [9]. Consistent with Faubel's data is the possibility that the apoptosis noted on day 2 resulted in secondary necrosis, which has been observed in cisplatin-induced renal tubular injury in vitro [2]. Additionally, it has been hypothesized that apoptotic and necrotic cell death represent two ends of a response continuum [18]. For example, it has been shown in vitro that shifts between the two forms of cells death may occur, depending on numerous variables, such as growth factors [19], oxygen/nutrient availability [20], adenosine triphosphate (ATP) concentration [21], and nitric oxide [22]. In cisplatin-induced ARF, many factors (e.g., ATP reduction) occur that have the potential to promote a shift from apoptotic to necrotic cell death [23].
IL-1α, like IL-1β, is a proinflammatory cytokine and initiates many of the same biological processes. However, IL-1α is unique in that it is not secreted from the cell and is rarely found in the extracellular compartment, even after activation. Because IL-1α exerts its proinflammatory actions either intracellularly or at the cell membrane [24], its functions are not inhibited by IL-1Ra. In one report, IL-1α was over-expressed in various cells in the presence of IL-1Ra, and, despite saturating concentrations of the latter, IL-1α activated NF-kB while AP-1 caused IL-8 and IL-6 release [24]. Presently, no specific inhibitor of IL-1α is available [25].
In this study, we also attempted to identify the mechanism by which IL-1α -/- mice are protected against cisplatin-induced ARF and to determine the role of proinflammatory cells in the disease. Thus, we compared the infiltration of proinflammatory cells, including neutrophils, CD11b-positive cells, as a marker of macrophages, and CD49b-positive cells, as a marker of pan-NK cells, in wild-type versus IL-1α -/- mice. Our results showed that there was no significant change in the infiltration of neutrophils or in CD11b- and CD49b-positive cells between wild-type and IL-1α -/- mice. According to our data and those of previous studies, neither neutrophil recruitment nor CD11b- and CD49β-positive cells are essential for the development of cisplatin-induced ARF. Unfortunately, we were unable to explain the mechanism underlying the protection of IL-1α -/- mice against cisplatin-induced ARF. Recently, Turner et al. [26] demonstrated that IL-1α stimulated human cardiac myofibroblasts to express proinflammatory cytokines, including IL-1b, TNF-α, and IL-6, via specific signaling pathways and that these responses are unaffected by IL-10 exposure. Additionally, TNF-α has been shown to play an important role in the pathogenesis of cisplatin-induced ARF [27]. Thus, important proinflammatory cytokines, such as IL-6 and TNF-α, merit further study in IL-1α -/- mice.
We observed that renal IL-1α increased early after cisplatin injection and that a deficiency of IL-1α attenuated renal dysfunction and tubular necrosis. However, no significant difference in neutrophil infiltration, or CD11b- and CD49b-positive cells was observed between wild-type and IL-1α -/- mice on day 3 after cisplatin administration.
In conclusion, IL-1α -/- mice are protected against cisplatin-induced ARF, mimicking the protection against cisplatin-induced ARF observed in caspase-1 -/- mice, which lack IL-1α. Further investigation of the mechanisms that protect IL-1α -/- mice against cisplatin-induced ARF is certainly warranted.
Acknowledgments
This work was supported by a grant from the 12th Asian Pacific Congress of Nephrology (2009).
Notes
No potential conflict of interest relevant to this article was reported.
