Effects of the Transition from Premenopause to Postmenopause on Lipids and Lipoproteins: Quantification and Related Parameters
Article information
Abstract
Background/Aims
The aim of this study was to quantitatively measure changes in lipids and lipoproteins during perimenopause and to identify variables related to these changes.
Methods
Among women who had three regular health evaluations over a span of 2-4 years, 34 women remained in the premenopausal state, 34 premenopausal women transitioned to the postmenopausal state, and 36 postmenopausal women were enrolled. The menopausal state was determined not only by a history of amenorrhea but also by levels of female sex hormones. Yearly changes in lipids were calculated using a linear regression of the three measurements.
Results
The transition from premenopause to postmenopause was associated with increased total cholesterol and low-density lipoprotein (LDL) cholesterol levels by 7.4 ± 8.0 mg/dL (4.2 ± 4.9%) and 6.9 ± 6.5 mg/dL (6.8 ± 7.0%) over one year, resulting in an elevation of 19.6 ± 22.6 mg/dL (10.9 ± 13.0%) and 18.9 ± 19.5 mg/dL (18.6 ± 20.3%), respectively, during perimenopause. There were no changes observed in premenopausal and postmenopausal women. Body weight, blood pressure, high-density lipoprotein (HDL) cholesterol, and triglycerides did not change in any of the three groups. In all women, changes in both total cholesterol and LDL cholesterol were associated with changes in follicle stimulating hormone (r = 0.40, p < 0.001 and r = 0.38, p < 0.001, respectively). Changes in triglycerides were associated with changes in body weight (r = 0.28, p = 0.005).
Conclusions
During perimenopause, total and LDL cholesterol levels increase and these changes in cholesterol are mainly dependent on changes in female sex hormones.
INTRODUCTION
Premenopausal women have distinctly less atherosclerotic cardiovascular diseases than men. After menopause, this incidence steeply increases, resulting in no difference between men and women in individuals older than 70. Therefore, menopause is considered as one of the risk factors for atherosclerotic diseases [1].
Many studies have shown that total cholesterol and low-density lipoprotein (LDL) cholesterol increase after menopause [2-11]. However, it is controversial as to what extent these atherogenic lipids are elevated. Cross-sectional studies have shown a considerable influence [2-4], whereas most longitudinal follow-up studies reveal only small changes [5-11]. It is also unclear whether the transition from premenopause to postmenopause leads to a decrease in high-density lipoprotein (HDL) cholesterol [7-11].
Changes in female sex hormones precede the cessation of menstruation [12]. Therefore, lipid profiles may start to change before menopause [11]. Since nearly all studies define postmenopausal status based on amenorrhea without measurements of female sex hormones [7], it is possible that these changes have been underestimated.
There are relatively few studies that have examined the mechanisms underlying changes in lipid profiles during the perimenopause. Many studies have observed the parameters affecting lipid levels [5-10,13]; however, the relationship of these variables with lipid levels was not evaluated.
In this longitudinal follow-up study, we investigated quantitative changes in lipid and lipoprotein profiles during the transition from premenopause to postmenopause, which were defined by both female sex hormones and amenorrhea history, and the parameters related to these changes.
METHODS
Women who had three regular health evaluations over the course of 2-4 years between 1999 and 2007 were screened. The regular health examination included the following: 1) history of medical illness, medication, and menstruation, 2) height and weight, 3) laboratory tests including complete blood count, urinalysis, routine blood chemistry, thyroid function tests, cancer markers, electrocardiogram, and chest X-ray, 4) ultrasound examination of the abdomen, and 5) esophagogastroduodenal endoscopy.
Among these women, 34 premenopausal women remained in the premenopausal state (premenopausal group), 34 premenopausal women transitioned to the postmenopausal state (perimenopausal group), and 36 postmenopausal women (postmenopausal group) were enrolled. The premenopausal state was defined as regular menstruation and follicle stimulating hormone levels (FSH) < 20 IU/L. The postmenopausal state was defined as amenorrhea ≥ 6 months and FSH levels ≥ 20 IU/L. Finally, the perimenopausal state was defined as the transition from premenopausal state to postmenopausal during follow-up.
Exclusion criteria included 1) diabetes mellitus, 2) hepatitis with aspartate aminotransferase or alanine aminotransferase levels ≥ 2-fold the upper normal limit, 3) other diseases that influence lipid levels such as infectious diseases, and 4) medications that affect lipid levels including female sex hormones and lipid lowering drugs during the follow-up period.
After overnight fasting, blood samples were obtained. Total cholesterol and triglycerides were determined by the enzymatic method using an automatic analyzer (Model 7150, Hitachi, Japan). HDL-C was measured by direct methods using an automatic analyzer. LDL-C was calculated using the Friedewald formula. FSH and estradiol were determined using radioimmunoassay (RIA) methods.
To minimize the biological variation in lipid levels, yearly changes in lipid levels were calculated from a linear regression line of the three measurements against age. Total change was obtained by multiplying yearly changes by the duration of follow-up.
Data are expressed as the mean ± SD. Statistical analyses were performed using SPSS version 9.0 (SPSS Inc., Chicago, IL, USA). An analysis of variance (ANOVA) or a Kruskal-Wallis test was used to evaluate differences among groups. The Friedman test was used to compare serial changes. Relationships among variables were analyzed using the Pearson correlation method. A stepwise linear regression method was used to identify independent parameters. The distribution of discrete variables was analyzed using a χ2 test. A two-tailed p value < 0.05 was considered statistically significant.
RESULTS
The perimenopausal group was younger than the postmenopausal group (p < 0.001) and older than the premenopausal group (p < 0.001, Table 1). The younger premenopausal group tended to be taller than the older postmenopausal group (p = 0.093). Other social histories, associated diseases, and the duration of the follow-up period were not different among groups.
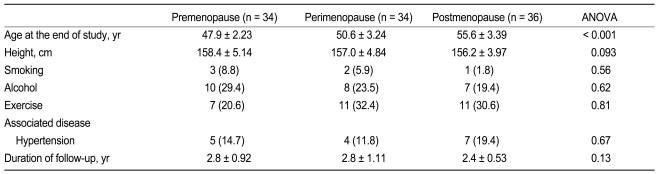
Comparison of baseline clinical characteristics among premenopausal women, perimenopausal women, and postmenopausal women
The transition from premenopause to postmenopause was associated with increased total cholesterol and LDL cholesterol levels by 7.4 ± 8.0 mg/dL (4.2 ± 4.9%) and 6.9 ± 6.5 mg/dL (6.8 ± 7.0%) over the course of one year (Fig. 1), resulting in a total elevation of 19.6 ± 22.6 mg/dL (10.9 ± 13.0%) and 18.9 ± 19.5 mg/dL (18.6 ± 20.3%) during the perimenopausal period, respectively (Table 2). Increases in total cholesterol and LDL cholesterol levels were greater in the perimenopausal group as compared with the premenopausal group (-0.64 ± 24.4 mg/dL [0.23 ± 12.3%], p = 0.002 and -0.74 ± 22.5 mg/dL [0.50 ± 18.2%], p < 0.001, respectively) and the postmenopausal group (5.17 ± 23.7 mg/dL [3.23 ± 11.6%], p = 0.009 and 6.29 ± 23.3 mg/dL [5.24 ± 25.5%], p = 0.007, respectively). Body weight, blood pressure, HDL cholesterol, and triglycerides did not differ in any of the three groups.

Comparison of yearly changes in lipid profiles among premenopausal women, perimenopausal women, and postmenopausal women. Mean ± SE; ap < 0.05; LDL-C, low density lipoprotein-cholesterol; HDL-C, high density lipoprotein-cholesterol.
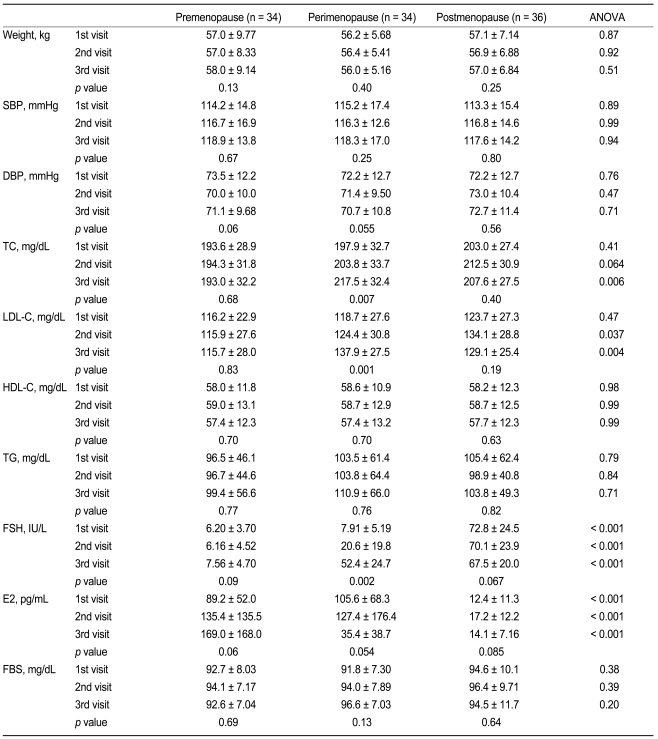
Changes in clinical and lipid profiles in premenopausal women, perimenopausal women, and postmenopausal women during the follow-up period
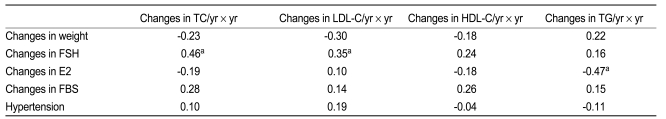
In all women (n = 104), changes in total cholesterol were positively associated with changes in FSH (r = 0.40, p < 0.001) (Table 3, Fig. 2) and negatively with changes in estradiol (r = -0.20, p = 0.046). In the multivariate analysis, FSH was an independent variable. Changes in LDL cholesterol were associated with changes in FSH (r = 0.38, p < 0.001), and this elevation was greater in women with hypertension (r = 0.25, p = 0.010) than in women without hypertension. An increase in triglycerides was related to changes in body weight (r = 0.28, p = 0.005) and was greater in women without hypertension (r = 0.19, p = 0.049). In the multivariate analysis, body weight was an independent variable.
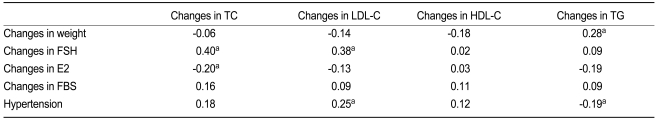
Relationship between changes in lipid levels (yearly change × follow-up year) with clinical and laboratory parameters in all women (n = 104)

Relationship between changes in cholesterol levels (yearly change × follow-up year) with changes in follicle stimulating hormone (FSH) in all women.
In perimenopausal women (n = 34) (Table 4), changes in both total cholesterol and LDL cholesterol were associated with changes in follicle stimulating hormone (r = 0.46, p = 0.006 and r = 0.35, p = 0.044, respectively). Changes in triglycerides were negatively associated with changes in estradiol (r = -0.47, 0.006).
DISCUSSION
This retrospective cohort study demonstrates that the transition from premenopause to postmenopause is associated with an increase in total cholesterol and LDL cholesterol levels and no change in HDL cholesterol levels. This elevation in atherogenic lipids was greater than those reported in previous follow-up studies. In addition, increases in total cholesterol and LDL cholesterol are related not to changes in body weight or fasting blood sugar, but mainly to changes in female sex hormones.
In the present study, changes in lipid levels were calculated using linear regression methods to minimize the influence of biological and analytical variations in lipid levels. However, changes in lipid levels calculated using this method were similar to those obtained via direct extraction of the first measurements from the last ones with a difference of < 3% (p > 0.05, data not shown).
Many studies have shown that total cholesterol and LDL cholesterol increase after menopause. In cross-sectional studies, total cholesterol was elevated by 9-19% and LDL cholesterol by 10-24% [2-4,14]. However, in longitudinal follow-up studies, total cholesterol was elevated by 5-14.2% and LDL cholesterol by 3.5-16% over the course of 2-6 years [5-10]. When changes in the control group, usually premenopausal women, were considered (2.6-9.6% for total cholesterol and 6.3-6.4% for LDL cholesterol), increases in total cholesterol and LDL cholesterol were only 2.4-7.2% and -2.9-9.7% respectively [5-10]. In the present study, total cholesterol and LDL cholesterol were elevated by 10.9% and 18.6%, respectively and changes in premenopausal women were negligible during the 2.8 years. Therefore, increases in atherogenic lipids observed in the present study were greater than those of previous studies. In one study that did not have a control group, total cholesterol and LDL cholesterol increased by 14% and 19%, respectively, from 4 years before to 1 year after menopause [11].
In almost all previous studies, menopausal status was determined only by amenorrhea history. Changes in female sex hormones precede the cessation of menstruation [12]. Women with regular menstruation and with altered female sex hormone levels may be assigned to the premenopausal state. However, atherogenic lipid levels have already begun to increase and these women have higher atherogenic lipid levels than premenopausal women with normal female sex hormone levels [11]. Therefore, changes during perimenopause may be underestimated and those in women remaining in the premenopausal state may be overestimated.
In addition, some women might be incorrectly categorized into the postmenopausal state because of amenorrhea due to other causes, even though female sex hormones are in the premenopausal state. In these women, atherogenic lipids have not yet increased. Therefore, if menopausal status is determined only by amenorrhea, it is possible that changes during perimenopause are underestimated and changes in postmenopausal women are overestimated. In the present study, menopausal state was determined not only amenorrhea history but also by female sex hormones. This difference in the definition of menopausal status might explain why the elevation in atherogenic lipids was greater in the present study, as compared with previous studies.
In the present study, enrolled women had extensive laboratory examinations and women with diseases affecting lipid levels were excluded. In contrast, most previous follow-up studies were dependent on interviews or questionnaires to evaluate health status. During the transition from premenopause to postmenopause, considerable numbers of women experience the onset of diseases affecting lipid levels. This difference in evaluating health status also partially explains the reason why the elevation in atherogenic lipids is greater in the present study than in previous studies.
It has been generally accepted that HDL cholesterol decreases after menopause, without solid evidence. In cross-sectional studies, changes in HDL cholesterol after menopause were relatively variable [2-4,14]. In follow-up studies, all studies, excluding a few, failed to demonstrate the decrease in HDL cholesterol after menopause [7-11]. In studies examining the effects of surgical menopause, HDL cholesterol did not decrease [15]. In the present study, the transition from premenopause to postmenopause was not associated with a decrease in HDL cholesterol. Therefore, loss of female sex hormones alone may not influence HDL cholesterol level.
To the best of our knowledge, there have been no reports that have identified variables associated with changes in lipid profiles during perimenopause. In almost all previous studies, parameters related to lipid levels, such as body weight or fasting blood sugar, did not change after menopause [5-10,13,16]. In the present study, these parameters were also unchanged. Changes in total cholesterol and LDL cholesterol were independently related to changes in FSH in both perimenopausal women and all women combined. These findings suggest that changes in total cholesterol and LDL cholesterol associated with menopause are mainly dependent on female sex hormones. Although changes in estradiol were also related to changes in lipid levels, this difference was not significant in the multivariate analysis. We believe that large biological variation may weaken the statistical power.
In all groups, LDL cholesterol increased more in women with hypertension than in women without hypertension (r = 0.25, p = 0.010). This phenomenon was independent of changes in female sex hormones.
Although triglyceride levels did not change with perimenopause, several variables were related to changes in triglyceride levels. However, factors related to changes in triglyceride levels were rather different to those related to changes in cholesterol levels. Changes in triglyceride levels were dependent both on body weight and female sex hormones, especially estradiol. In all women, changes in body weight were the most important variable (r = 0.28, p = 0.005). Changes in triglyceride levels were negatively associated with changes in estradiol levels in perimenopausal women (r = -0.47, p = 0.006) and in all women with borderline significance (r = -0.19, p = 0.054). Because the administration of estrogen to postmenopausal women increases triglyceride levels [17-19], changes in triglycerides may not be a direct effect of changes in female sex hormones, but secondary to the processes associated with the loss of estradiol. We cannot explain this phenomenon and further studies are necessary.
There are several limitations to the present study. The number of patients in each group was small and the mean age was different among the groups. Since changes in lipid levels were similar between younger premenopausal and older postmenopausal women, we believe that this difference did not significantly influence the results. This study enrolled women who were relatively wealthy and highly educated, since regular health examinations are expensive. Therefore, the finding may not be applicable to the general population. In the present study, total and LDL cholesterol levels were higher in postmenopausal women transiting from the premenopausal status than in initially postmenopausal women. We think that this finding results from the exclusion of postmenopausal hypercholesterolemic women taking lipid-lowering drugs.
The present study demonstrates that total and LDL cholesterol levels increase much more than observed in previous reports, and this elevation is mainly dependent on changes in female sex hormones during perimenopause. Since cardiovascular disease increases after menopause [1] and hormone replacement therapy is ineffective for prevention [20], close monitoring and intensive management using cholesterol-lowering drugs is necessary for perimenopausal women. HDL cholesterol levels are not changed. Although triglyceride levels did not change during perimenopause, changes in triglyceride levels were associated with changes in body weight.
Acknowledgements
This Research was supported by Chung-Ang University Research Grants in 2009.
Notes
No potential conflict of interest relevant to this article was reported.