Cultural Adaptation of a Compliance Questionnaire for Patients with Rheumatoid Arthritis to a Korean Version
Article information
Abstract
Background/Aims
The Compliance Questionnaire-Rheumatology (CQR) is a validated scale to evaluate patient compliance for anti-rheumatic medications. We developed a Korean version of the CQR (KCQR) and confirmed its reliability and validity.
Methods
We prepared the KCQR by translating and back-translating the original CQR with modifications to adapt it to Korean culture. Fifty Korean patients with rheumatoid arthritis (RA) were enrolled in this study. The test-retest reliability of the KCQR was evaluated at a 2-week interval using the intraclass correlation coefficient (ICC). The validity of the KCQR was assessed by identifying associations between KCQR scores and patient compliance, measured using pharmacy refill data.
Results
The reliability of the KCQR was adequate, with an ICC of 0.71 for test-retest reliability. With respect to validity, the summed score of the weighted KCQR showed a significant correlation with pharmacy refill data (r2 = 0.57) on multiple regression analysis.
Conclusions
Our results indicate that the KCQR is a reliable, valid instrument to evaluate compliance of Korean patients for RA medications.
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic inflammatory disease, causing chronic inflammation of the joints and various organs. Despite the recent development of different agents, the long-term use of anti-rheumatic drugs is still an essential part of the management strategies for patients with RA [1,2]. Medication compliance is of particular importance in patients who suffer from chronic diseases such as RA, because these patients are easily discouraged by the prospect of lifetime treatment with imperfect anti-rheumatic drugs [3]. It has been reported that greater compliance leads directly to enhanced clinical effects [4,5]. Noncompliance causes several critical problems in the management of these patients, including unnecessary health costs, changes in treatment, morbidity, and potential mortality [6]. Thus, measuring and predicting the compliance of a particular patient is a useful tool for optimal patient management.
Medication compliance can be quantified in several ways [7], including direct and indirect measures of ingestion, clinical measures, and patient self-reporting [8]. Direct measurement of ingestion involves quantifying the concentrations of substances in body fluids. Indirect measurements include monitoring pharmacy refills, tablet counts, and the use of electronic devices and questionnaires. Clinical measures require clinical judgment and using therapeutic outcome to infer the degree of compliance. Obviously, patient self-reporting depends solely on the patient.
Using a validated compliance questionnaire has several advantages over the other methods. It can be done easily without requiring an invasive procedure, it is inexpensive, and it provides relatively accurate measures of true compliance and can encourage real-life compliance. Furthermore, it can predict a particular patient's compliance, not just measure compliance results [8-10]. There are at least two English-language compliance questionnaires for rheumatologic diseases [11,12]. However, there is no validated compliance questionnaire written in Korean in widespread use. Thus, we developed a Korean version of a compliance questionnaire, based on the Compliance Questionnaire-Rheumatology (CQR), a validated scale for comparing self-reported compliance and electronic medical device findings [12,13].
METHODS
Patients
Fifty Korean patients with RA who agreed to take part in the study were enrolled from the Rheumatologic Clinic of Seoul National University Hospital. All patients met the classification criteria for RA of the American College of Rheumatology [14].
The Institutional Review Board of Seoul National University Hospital approved this study. Appropriate informed consent was obtained.
Translation of the CQR
The CQR was translated into Korean by three translators, and an initial draft KCQR was prepared by one of the authors (SYL), and was then translated back into English by the other three authors (JYL, HJH, EBL). During the translation/back-translation procedure, one question was modified. Because medication organizers are not popular in Korea, the sentence "I use a dose organizer for my medications" was changed to "I use a special way so that I don't forget to take my anti-rheumatic medicines, like a pocketbook, calendar, or alarm clock". A four-point Likert scale was adopted with the anchors "don't agree at all" (score = 1), "don't agree" (score = 2), "agree" (score = 3), and "agree very much" (score = 4). To check the appropriateness of the translated questionnaire, we conducted a field test in seven patients. The results of the field test led to the revision of four items on the questionnaire to enhance patient comprehension. One native American who is bilingual in English and Korean reviewed the final result. The final version of the KCQR is summarized in Figure 1.
Reliability
Reliability was tested using the test-retest method performed with a 2-week interval between the two processes. The first test was done when the patient visited the clinic. The questionnaire was mailed to their homes 1 week later. Two weeks after they had taken the first test, the patients were telephoned and asked to open and complete the questionnaire. Thirty-six patients completed the retest and returned completed questionnaires.
Validity
The validity of the KCQR was assessed by comparing the KCQR scores and compliance as measured by pharmacy refill data (refill compliance). The following medications were included for validity analysis: systemic non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs), and antiulcer medications. On-demand medications, topical preparations, and medications prescribed transiently were excluded from the pharmacy refill analysis. Pharmacy refill data for 41 patients who took medication for more than 6 months were obtained.
In Korea, the National Health Insurance Review Agency (HIRA) governs health insurance for all Koreans. A doctor diagnoses a patient and issues a prescription. With the prescription in hand, the patient visits a drugstore to get the medicine. In this study, pharmacy refill data were obtained in two steps. After obtaining prescription refill data from Seoul National University Hospital, the final pharmacy refill data were completed with dispensation records from drugstores, which were obtained from HIRA. Refill compliance was calculated as (total dose of medication prescribed and dispensed)/(total number of prescribed doses) × 100%.
Statistical analyses
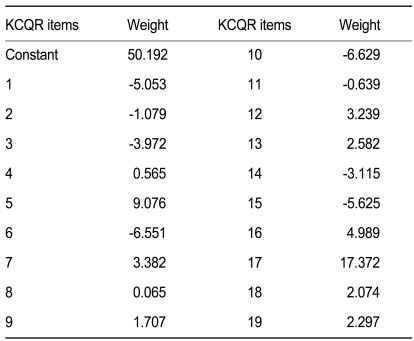
The KCQR score was calculated as the sum of 19 scored items. Some of the items in the CQR received a negative score if the patient indicated less compliance. These items were recoded (score 1→4, 2→3, 3→2, 4→1) to obtain a useful total score, where high scores are related to higher compliance. Test-retest reliability was measured by calculating the intraclass correlation coefficient (ICC), a measure of concordance that corrects for systemic errors [15]. To evaluate validity, the KCQR scores and refill compliance were analyzed using Pearson correlation coefficients. Multiple regression analyses were used to obtain the weights that increased the correlation between KCQR score and refill compliance, as previously reported [13]. All analyses were performed using the SPSS version 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics
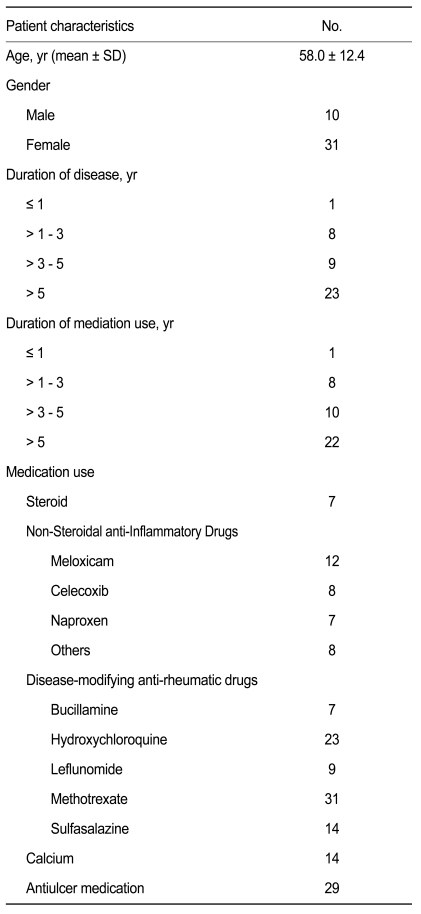
The baseline characteristics of the 41 patients who entered into the final analysis are summarized in Table 1. The mean (± standard deviation) age of the patients was 58.0 (± 12.4) years. There were 31 females and ten males. Twenty-three patients (56.1%) had a disease duration exceeding 5 years, while five patients had a disease duration less than 1 year. The duration of medication was similar to the disease duration.
Reliability
Of the 50 patients who completed the first test, 36 patients (72.0%) returned the mailed retest questionnaire. The average score (± SD) was 61.39 (± 5.97) on the first measurement and 61.50 (± 6.84) 2 weeks later. The test-retest reliability gave an intraclass correlation coefficient of 0.71 (95% confidence interval [CI], 0.50 to 0.84) for the KCQR score.
Validity
Compliance as measured using the record of pharmacy refills was 89.4 ± 14.5%. The total KCQR score, which was the sum of the unweighted individual items, showed a low correlation with refill compliance (r2 = 0.13, p = 0.020) (Fig. 2). However, when individual items were weighted with the values inferred from the multiple regression analysis (Table 2), the KCQR showed significant correlation with refill compliance (r2 = 0.57) (Fig. 3), indicating that weighting is an essential step in interpreting the KCQR.
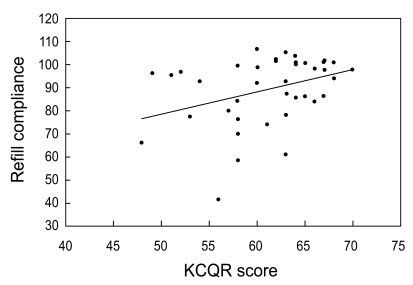
Correlation between refill compliance and the Korean version of the Compliance Questionnaire-Rheumatology (KCQR) score (r2 = 0.13, p = 0.02). Refill compliance was obtained from pharmacy refill data for 41 patients who took medication for more than 6 months.
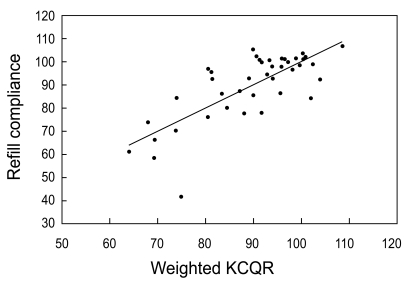
Correlation between refill compliance and the weighted Korean version of the Compliance Questionnaire-Rheumatology (KCQR) score (r2 = 0.57, p < 0.001). Refill compliance was obtained from pharmacy refill data for 41 patients who took medication for more than 6 months. Multiple regression analyses were used to obtain the weights that increased the correlation between the KCQR score and refill compliance.
DISCUSSION
In this study, we developed a Korean version of the CQR. Our KCQR showed adequate reliability (ICC = 0.71) for clinical application. The validity of the instrument, which was calculated from refill compliance, was also adequate for clinical use (r2 = 0.57).
The study of the original CQR measured compliance in patients with RA, polymyalgia rheumatica, and gout [13]. However, we restricted our study to patients with RA because the immediate clinical effects of non-compliance in RA and gout are different. Non-compliance in RA can lead to brief aggravation of arthralgia, while there is no such immediate effect in gout. Polymyalgia rheumatica is very rare in Koreans.
Pharmacy refill data were used to infer the true value of compliance in this study. A direct chemical measurement method was excluded because measurement methods have not been established for some medications. Of the indirect measurement methods, we could not accept tablet counts or electronic monitoring methods because they affect real-life compliance. There are many reports that using an electronic monitoring device can increase compliance [16-22]. Clinical compliance was inadequate because most of the enrolled patients were those of one of the authors (EBL). Some authors have suggested that self reporting is a useful measurement technique, if the client is interviewed at home [23-25]. However, this still has the inherent limitation that it is purely subjective. Refill compliance based on pharmacy refill data is a method that best reflects real-life compliance. The unique Korean health insurance system, administered by one nationwide government agency, enabled us to measure pharmacy refill data accurately. Because all drugstores in Korea are reimbursed by one agency, it is possible to trace all dispensation data from all drugstores. Thus, we used refill compliance as a surrogate for true compliance. The compliance of Korean RA patients as measured by pharmacy refill data in this study was 89.4 ± 14.5%, which is comparable to other studies [13], suggesting that the estimation of true compliance in our study was adequate.
The test-retest reliability yielded an intraclass correlation coefficient of 0.71 for the KCQR score, which is comparable to the original CQR study (0.73) [13]. The simple sum of the KCQR correlated weakly with pharmacy refill compliance, whereas the weighted sum of the KCQR correlated adequately. Two highly weighted questions were #16 (I use a dose organizer for my medications) and #17 (What the doctor tells me, I remember). This study changed the former to "I use a special way so that I don't forget to take my anti-rheumatic medicines, like a pocketbook, calendar, or alarm clock". Question #5 (My medicines are always stored in the same place, and that's why I don't forget them) also addresses the concept that patients may have established a special way to remember to take their anti-rheumatic medications and was also weighted heavily. The answers to these questions, especially questions #16 and #5, proved valuable when determining the compliance in RA patients. Doctors and pharmacists should advise patients to keep their medicines in the same place each day and develop a special way to remember to take them.
The KCQR has many advantages over other compliance-measuring methods. It is simple and can be administered easily without the help of an investigator. The questionnaire can be completed within 10 minutes. As we demonstrated, it has good reliability and validity. A distinct merit of this questionnaire is that it can be used to predict the level of compliance before medication is started. This is very useful for devising treatment plans for individual patients. Additionally, most clinical trials of new anti-rheumatic drugs require long periods of participation by patients, as most rheumatic diseases require long-term treatment. In this respect, the KCQR may be used to screen participants for their potential participation in such studies.
This study analyzed 41 of the 50 patients enrolled. Despite a suggestion that 30 patients are enough for cultural adaptation studies [26], more patients may give more reliable results. This limitation needs to be considered when interpreting the results.
In conclusion, we developed a Korean version of a compliance questionnaire for rheumatoid arthritis. It showed adequate reliability and validity for clinical application compared to international studies. However, more studies are warranted to develop an instrument specifically tailored to Korean patients with RA.
Acknowledgements
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry for Health and Welfare, Republic of Korea (A030001, A084204).
Notes
No potential conflict of interest relevant to this article was reported.