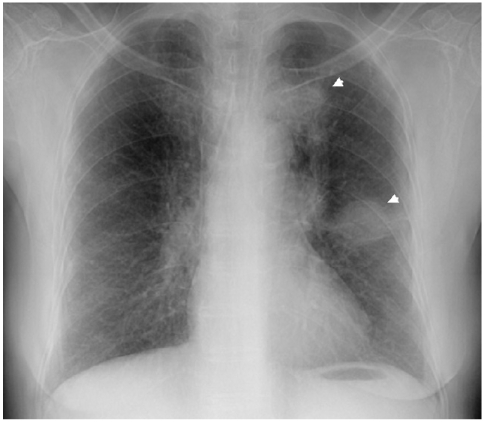
Two Lung Masses with Different Responses to Pemetrexed
Article information
Abstract
We described here a patient who had two lung masses. Although the two masses had the same histology and a similar good response to initial chemotherapy with gemcitabine and carboplatin, the response to pemetrexed as a second-line treatment was different after re-growth of the tumors. These two lung masses could have originated from different clones or they could have progressed through different paths of molecular pathogenesis after metastasis, which would lead to different tumor characteristics, including their chemosensitivity. Regardless of their pathogenetic mechanisms, it seems important to recognize that tumors with the same histology that develop in one patient can have different responses to drugs.
INTRODUCTION
Multiple synchronous lung carcinomas are not uncommon and the incidence of this malady ranges from 1% to 7% [1]. Because the prognosis has generally been poor for cases with lung metastases [2], it is critical to distinguish synchronous primary cancer from intrapulmonary metastases. Martini and Melamed [3] indicated that synchronous lung cancer requires the following criteria for diagnosis: 1) the two tumors must be anatomically distinct and separate, 2) the tumors are historically different, and 3) if the tumors' histology is of the same type, then each tumor should have its own original site, and there should be no cancer in the lymphatic vessels common to both, and no metastasis from another organ. However, it is often not so easy to differentiate multiple primary cancers from single primary cancer with metastasis, and especially in cases where two lesions have the same histology. We report here on a unique case that had two distinct lung masses with the same location, the same histology and a similar good response to initial chemotherapy with gemcitabine and carboplatin, but these tumors showed a different response to pemetrexed as a second-line treatment.
CASE REPORT
A 74-year-old woman came to our hospital for evaluation of her hemoptysis. Two distinct 3 cm-sized lung masses in the left upper lobe were found on the chest PA (Fig. 1). Squamous cell carcinoma was diagnosed by trans-thoracic needle biopsy of the lower mass. Although the other mass could have been a double primary carcinoma, further diagnostic tests were not done because the patient did not want them at that time. There was also enlargement of multiple mediastinal lymph nodes, suggesting that any benefit from radical treatment would not be great. After four cycles of chemotherapy with gemcitabine (1,000 mg/m2, days 1 and 8, every 3 weeks) and carboplatin (area under the curve: 6, day 1, every 3 weeks), the size of the two lung masses was decreased and so this was deemed a partial response. During the subsequent follow-up, the lung masses were again increased in size and she received second-line chemotherapy with two cycles of pemetrexed (500 mg/m2, day 1, every 3 weeks). After completion of chemotherapy with pemetrexed, the lower mass was markedly resolved while the upper mass did not respond (Fig. 2). Biopsy to the other mass was performed to determine whether it was a double primary carcinoma. However, the histology was almost same as the previously obtained specimen, and this made it difficult to differentiate the mass from metastasis (Fig. 3). Considering her age and performance, we decided not to administer further chemotherapy and so we recommended regular follow-up.
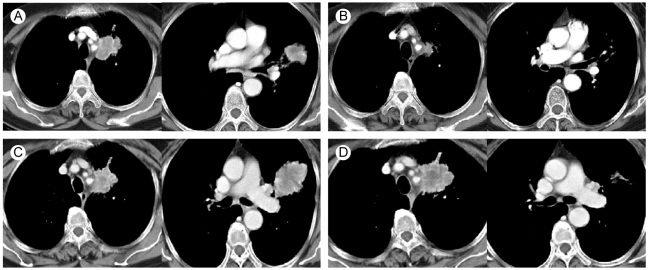
(A) Two lung masses in the left upper lobe. (B) The masses after treatment with gemcitabine and carboplatin. (C) Increased size of the masses during follow-up. (D) Different response of the two masses to pemetrexed.

The histologic findings were diagnosed as squamous cell carcinoma; the tumor was composed of cells arranged in sheets with irregular nuclei and a large amount of cytoplasm. The individual specimens from the lower mass (A) and the upper mass (B) showed similar histological features (Papanicolau stain, × 200).
DISCUSSION
The rate of detecting synchronous multiple lung tumors has recently increased due to the advances in diagnostic imaging modalities such as multi-slice spiral computed tomography and positron emission tomography scanning. Most reported cases of synchronous tumors in the lung have been shown to have the same histologic type. Among the possible tumor combinations reported in the medical literature, squamous cell carcinoma was by far the most common [1,3,4]. Thus, having the same cell type cannot exclude the possibility of synchronous double primary carcinoma. Various methods like DNA flow cytometry and DNA marker analysis can provide information about the loss of heterozygosity, and this might be useful to accurately identify genetic difference of two masses that have the same histology [1,5]. In our case, we could not carry these procedures out because the amounts of the procured tissue samples were too small.
The two lung masses of this patient could have originated from different clones or they could have progressed through different paths of molecular pathogenesis after metastasis, which would lead to different tumor characteristics, including their chemosensitivity. Regardless of their pathogenetic mechanisms, it seems important to recognize that tumors with the same histology that develop in one patient can have different responses to drugs. We previously reported on another case of multiple primary lung cancers that had different responses to gefitinib. In that case, the sensitivity to gefitinib was related with mutations of epidermal growth factor receptor-tyrosine kinase [6].
Pemetrexed is a multi-targeted antifolate that inhibits several folate-dependent enzymes involved in both purine and pyrimidine synthesis. The overall response rate was 9.1% for pemetrexed in a randomized phase III trial of pemetrexed versus docetaxel in patients with non-small cell lung cancer and who had been previously treated with chemotherapy [7]. In 2007, there was a report of the in vitro chemosensitivity of freshly explanted tumor cells to pemetrexed. In that study, a low level of thymidylate synthase (TS) and the mrp4 gene expression significantly correlated with the chemosensitivity to pemetrexed, and the predictive value of TS and the mrp4 gene expression was independent of the tumor type [8]. This might contribute to explaining the different responses to pemetrexed in our case, although this needs to be confirmed by further investigations.
Predicting the chemosensitivity of individual patients is important for improving the efficacy of cancer treatment and avoiding unwanted side effects and the economical expense of ineffective agents. However, this is quite challenging because drug responses reflect both the characteristics of the targeted cells, the mode of drug delivery and/or the host's metabolism. Although several genes have been reported to determine the response to drugs [9], this seems to be useful in only very limited situations. In addition, several attempts have been made to predict drug sensitivity with transcriptional profiling by using c-DNA microarray, proteomic profiling, in vitro culture assay etc [9-11]. Even though previous studies have shown some feasibility to achieve this prediction of response, the practical application to actual clinical cases still seems to have a long way to go.
We currently don't know what determines the response to pemetrexed. However, we believe this can be clarified in the near future via efforts to gain a better understanding about carcinogenesis and to find more molecular factors that can affect the sensitivity to drugs. Gaining such knowledge may lead to a new era of individualized/tailored cancer therapy.
Notes
No potential conflict of interest relevant to this article was reported.