The Impact of Generic Clopidogrel Bisulfate on Platelet Inhibition in Patients with Coronary Artery Stents: Results of the ACCEL-GENERIC Study
Article information
Abstract
Background/Aims
In patients with coronary artery stents, the cost of clopidogrel has been cited as a factor in the premature discontinuation of therapy. Thus, the introduction of lower-cost generic clopidogrel may increase patient compliance. However, platelet inhibition by generic clopidogrel has not been compared to the original clopidogrel formulation in patients with coronary artery stents.
Methods
We prospectively enrolled 20 patients receiving chronic therapy with the original clopidogrel bisulfate (Plavix®). After assessing patient compliance with Plavix®, maintenance therapy was switched to generic clopidogrel bisulfate (Plavitor®). Platelet reactivity was assessed at baseline and 30-day after the switch using conventional aggregometry and the VerifyNow P2Y12 assay.
Results
All patients completed maintenance therapy with Plavitor®. Before and after switching therapy maximal (36.5 ± 7.9% vs. 39.8 ± 16.2%, p = 0.280) and late platelet aggregation (23.5 ± 10.9% vs. 29.1 ± 18.3%, p = 0.156) with 5 µmol/L adenosine diphosphate (ADP) stimulus did not differ. Likewise, 20 µmol/L ADP-induced platelet aggregation and P2Y12 reaction unit in patients on Plavitor® therapy was comparable to that in patients on Plavix® therapy. However, Bland-Altman analysis showed wide limits of agreement between measured platelet reactivity on Plavix® vs. Plavitor® therapies.
Conclusions
Among patients on Plavix® maintenance therapy with coronary stents, replacement with Plavitor® shows a comparable inhibition of ADP-induced platelet aggregation. However, due to poor inter-therapy agreement, between two regimens, physicians may be cautious when introducing generic clopidogrel bisulfate.
INTRODUCTION
Antiplatelert therapy that combines thienopyridine and aspirin has reduced the incidence of ischemic cardiovascular events in patients with percutaneous coronary intervention (PCI) or acute coronary syndrome (ACS) [1-5]. Because the introduction of drug-eluting stents (DES) has significantly decreased neointimal hyperplasia and the need for repeated procedures due to restenosis compared to baremetal stents. DESs are now used in high-risk lesions [6-8]. However, DES have been associated with delayed endothelialization [9,10], localized hypersensitivity reactions [11], endothelial dysfunction [12], and late stent thrombosis (ST) [13-15].
Premature discontinuation of thienopyridine therapy has been associated with a marked increase in the risk of ST [16-19]. The Ameican Heart Association, American College of Cardiology, Society for Cardiovascular Argiography for Interventions, American College of Surgeors, and American Dental Association Science Advisory has stressed the importance of at least 12 months of dual antiplatelet therapy after DES implantation, along with educating the patient and healthcare providers about the hazards of premature discontinuation [20]. Because the cost of clopidogrel has been cited as a factor in the discontinuation of therapy [21], the introduction of lower-cost generic clopidogrel may significantly increase patient compliance. Because, clopidogrel hyporesponsiveness or high post-clopidogrel platelet reactivity (HPPR) has been associated with untoward clinical events [22-25], adenosine diphosphate (ADP) P2Y12 blockade by generic clopidogrel must be as effective as that of original clopidogrel. However this has not been demonstrated in patients with coronary artery stents. We thus addressed this concern by performing a prospective study comparing the degree of platelet inhibition by original (Plavix®, Sanofi-Aventis, Paris, France) versus generic (Plavitor®, Dong-A Pharmaceutical, Seoul, Korea) clopidogrel bisulfate in patients receiving dual antiplatelet therapy PCI-treated.
METHODS
Patient selection
Patients were eligible for enrollment if they were ≥ 18 years of age, previously treated with DES implantation for documented coronary artery disease, and were receiving clopidogrel bisulfate treatment at a steady state dose of 75 mg/day for at least 6 months. We enrolled patients from cohorts admitted for follow-up coronary angiography. Major exclusion criteria included repeated PCI after follow-up coronary angiography; coronary stenting due to a high-risk complex lesion; active bleeding and bleeding diatheses; oral anticoagulation therapy with warfarin; left ventricular ejection fraction < 30%; leukocyte count < 3,000 /mm3; platelet count < 100,000 /mm3; aspartate aminotransferase or alanine amino-transferase level ≥ 3 times upper normal; stroke within 3 months; non-cardiac disease with a life expectancy < 1 year; and inability to follow the protocol. The study protocol was approved by the Institutional Review Board, and the patients provided written informed consent for participation.
Study design
The ACCEL-GENERIC (platelet inhibition After Change of ClopidogrEL bisulfate with GENERIC tablet) study is a prospective, controlled, platelet function study of patients with coronary artery stents. A diagram of the study protocol is illustrated in Fig. 1. All patients were treated with DES, followed by 75 mg/day Plavix® for at least 6 months. We evaluated patient compliance by interview and tablet counting and enrolled patients showing complete compliance for at least 1 month. Immediately after insertion of the radial sheath for follow-up coronary angiography, blood samples for residual platelet reactivity analyses were obtained. Coronary angiography was performed according to standard techniques. If patients met the enrolled criteria and gave permission, Plavix® was replaced with 75 mg/day of Plavitor®. At the 30-day follow-up visit, patient compliance to antiplatelet therapy was assessed by interview and tablet counting. Blood samples for platelet function testing were obtained at 30 days after Plavitor® replacement, 2 to 4 hours after the last intake of the study medication. Peripheral venous blood samples were drawn from an antecubital vein using a 21-gauge needle.
Platelet function measurements
Blood samples were collected using the double-syringe technique, in which the first 2 to 4 mL blood is discarded to avoid spontaneous platelet activation. Platelet function was measured by light transmittance aggregometry (LTA) and the VerifyNow P2Y12 assay (Accumetrics, San Diego, CA, USA).
Platelet aggregation was assessed by LTA according to a standard protocol [26]. Briefly, blood samples were drawn into Vacutainer tubes containing 0.5 mL sodium citrate 3.2% (Becton-Dickinson, San Jose, CA, USA) and processed within 60 minutes. Platelet-rich plasma (PRP) was obtained as a supernatant fluid after centrifuging blood at 120 g for 10 minutes. The remaining blood was further centrifuged at 1,200 g for 10 minutes to prepare platelet-poor plasma (PPP). PRP was adjusted to platelet counts of 250,000/µL by adding PPP as needed. Platelet aggregation was assessed at 37℃ using an AggRAM aggregometer (Helena Laboratories Corp., Beaumont, TX, USA). Light transmission was adjusted to 0% with PRP and to 100% with PPP for each measurement. Platelet functions were measured after adding 5 and 20 µmol/L ADP, and curves were recorded for 10 minutes. Platelet aggregation was measured at peak (Aggmax) and at 5 minutes (Agglate) by laboratory personnel blinded to group assignment. Aggmax is considered to reflect the activity of both P2Y1 and P2Y12 receptors, whereas Agglate is indicative of P2Y12 receptor activity. The percentage of platelet disaggregation between Aggmax and Agglate was defined as follows: disaggregation (%) = [(Aggmax - Agglate) / (Aggmax)] × 100 [26].
The VerifyNow P2Y12 assay is a whole-blood, point-of-care (POC) system, which was developed to assess responsiveness to clopidogrel and other P2Y12 antagonists [26]. Blood was drawn into a Greiner Bio-One 3.2% citrate Vacuette tube (Greiner Bio-One, KremsmÜnster, Austria). The assay device consists of whole-blood assay channels. One contains fibrinogencoated polystyrene beads and 20 µmol/L ADP. This channel also contains 22 nmol/L PGE1 to reduce the nonspecific contribution of P2Y1 receptors. Results are reported in P2Y12 reaction units (PRU). We have previously correlated the two methods in our laboratory [27].
End point definition
The primary end point was ADP-induced Aggmax at baseline and 30-day after the switch to Plavitor®. The secondary end points were ADP-induced Agglate, percentages of platelet disaggregation, and PRU at baseline and 30-day after the switch.
Statistical analysis
Sample size was selected on the basis of practical consideration, and the trial was designed to be a pilot study in accordance with the paucity of platelet function tests directly comparing Plavix® and Plavitor®. Continuous variables are presented as mean ± standard deviation (SD) and compared using Student paired t, Wilcoxon signed-rank, or Mann-Whitney U tests. Categorical variables are presented as numbers or percentages and were compared using chi-square or Fisher exact tests (if an expected frequency was < 5). Agreement of platelet function measurements between baseline and 30-day after switch to Plavitor® was assessed using the Bland-Altman analysis. This analysis measures bias, which shows the systematic error responsible for either underor overestimation of a value, and sets the limits of agreement between the Plavix® and Plavitor® measurements. A p value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 13 (SPSS Inc., Chicago, IL, USA).
RESULTS
Patient characteristics and follow-up
Platelet function measurements in patients taking 75 mg/day of Plavix® were performed in a total of 20 patients (Table 1). These patients received Plavix® for 271 ± 81 days. Because treatment with Plavitor® was well tolerated and no subject discontinued the study drugs, platelet function 30 days after replacing medications was assessed in all patients. The number of remaining tablets demonstrated complete compliance with the study protocol. There were no cardiovascular events, and no major or minor bleeding noted.
Primary end point
Aggmax values after 30 days of Plavitor® therapy were similar to those after chronic Plavix® administration (Fig. 2). Aggmax with 5 µmol/L ADP stimulus was 39.8 ± 16.2% on Plavitor® therapy and 36.5 ± 7.9% on Plavix® therapy, with a mean difference of 3.3% (95% confidence interval [CI], - 2.9 to 9.4; p = 0.280). When Aggmax was assessed after stimulation with 20 µmol/L ADP, Plavitor® therapy achieved a similar platelet aggregation relative to Plavix® therapy (54.1 ± 16.0% vs. 52.8 ± 9.8%), with a mean difference of 1.3% (95% CI, - 4.9 to 7.5; p = 0.667).
Secondary end points
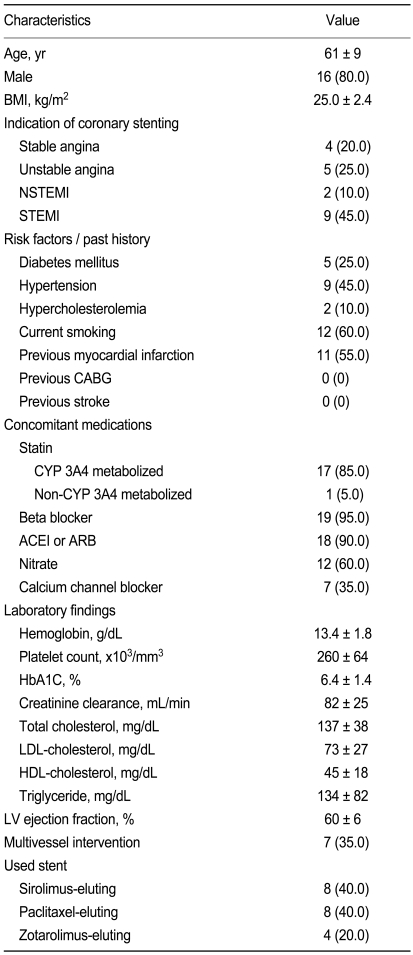
Significant changes in Agglate after 30-day of Plavitor® therapy were not observed compared to Plavix® therapy (Fig. 3A). Agglate with 5 µmol/L ADP stimulation was 29.1 ± 18.3% on Plavitor® therapy and 23.5 ± 10.9% on Plavix® therapy, with a mean difference of 5.6% (95% CI, - 2.3 to 13.5; p = 0.156). When Agglate was assessed after stimulation with 20 µmol/L ADP, platelet reactivity in patients on Plavitor® therapy was similar to that in patients on Plavix® therapy (42.7 ± 21.7% vs. 40.1 ± 15.9%), with a mean difference of 2.6% (95% CI, - 5.5 to 10.6; p = 0.518).
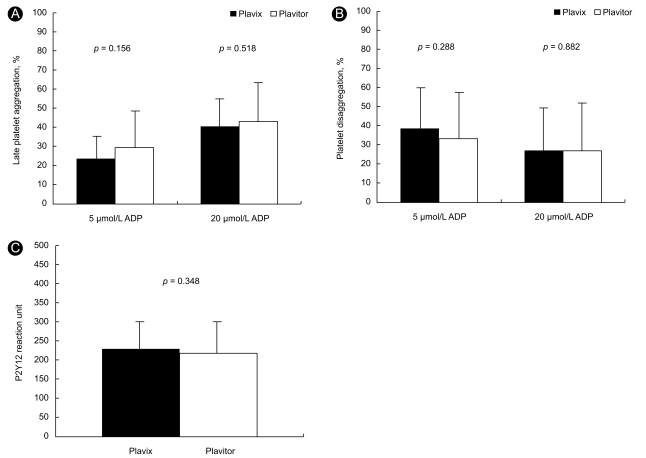
Comparison of late platelet aggregation (A) platelet disaggregation (B) and P2Y12 reaction unit (C) on Plavix® versus Plavitor® therapies. Bars indicate standard deviations. ADP, adenosine diphosphate.
A significant change in platelet disaggregation after 30-day of Plavitor® therapy was not identified compared to Plavix® therapy (Fig. 3B). Platelet disaggregations in patients on Plavitor® therapy were similar to those in patients on Plavix® therapy after stimulation with 5 µmol/L ADP (33.1 ± 22.2% vs. 38.5 ± 21.3%; 95% CI, - 15.5 to 4.9; p = 0.288) and 20 µmol/L ADP (26.6 ± 23.6% vs. 27.1 ± 21.4%; 95% CI, - 7.5 to 6.5; p = 0.882).
Using the VerifyNow P2Y12 assay, PRU values in samples from patients who had received the two therapies were comparable (Fig. 3C):Plavitor® therapy of 218.4 ± 75.2 so Plavix® therapy of 229.2 ± 57.7 (95% CI, - 12.7 to 34.3; p = 0.348).
Agreement of platelet reactivity between Plavix® and Plavitor® therapy
We conducted a Bland-Altman analysis to evaluate the agreement between platelet function measurements after Plavix® or Plavitor® therapy. We first compared Aggmax values after stimulation with 5 and 20 µmol/L ADP. Intertherapy analysis of 5 µmol/L ADP-induced Aggmax values showed a bias of - 3.3%, with a lesser Aggmax on Plavix® therapy (Fig. 4A). However, the limits of agreement varied from - 29.0 to 22.5%, indicating that the two therapies may significantly disagree in certain individuals. Furthermore, 20 µmol/L ADP-induced Aggmax values on Plavix® and Plavitor® therapies indicated poor accord with a slight bias of - 1.3% and wide limits of agreement ranging from - 27.4 to 24.8% (Fig. 4B). Compared to PRU on Plavitor® therapy, that on Plavix® therapy showed a bias of 10.8% and wide limits of agreement ranging from - 87.5 to 109.1 (Fig. 4C). Agglate values after stimuli with 5 and 20 µmol/L ADP also showed poor accord between Plavix® and Plavitor® therapies (Fig. 4D and 4E).
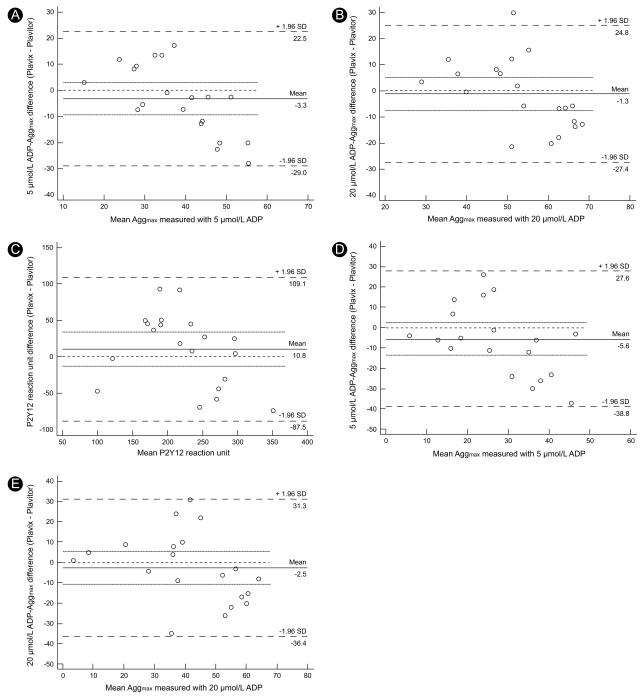
Bland-Altman agreement analysis in assessing residual platelet reactivity on Plavix® versus Plavitor® therapies. 5 and 20 µmol/L ADP-induced maximal plateler aggregation (A and B), P2Y12 reaction unit and 5 and 20 µmol/L ADP-irduced late plateler aggregation (B and E). Solid line represents bias, dotted lines represent 95% confidence interval of bias, and dashed lines represent limits of agreement. ADP, adenosine diphosphate; Aggmax, maximal platelet aggregation; Agglate, late platelet aggregation.
DISCUSSION
This, ACCEL-GENERIC study compared residual platelet reactivity measured by LTA and the POC VerifyNow assay after therapy with original versus generic clopidogrel bisulfate. There were no inter-therapy differences in residual platelet reactivity in patients treated with coronary stents. However, inter-therapy agreements were poor when comparing platelet function measurements, indicating that there might be a risk of clinical events in some cases after switching to generic clopidogrel bisulfate.
In-vitro platelet function assays have revealed response variability in platelet inhibition with the standard clopidogrel dose [28]. Furthermore, high residual platelet reactivity contributes to adverse clinical outcomes, including ST after stenting or in ACS patients [22-25]. Adequate inhibition of ADP-induced platelet aggregation by P2Y12 antagonists may contribute to decreased rates of ischemic clinical events.
Studies have investigated whether platelet reactivity events accrue after a cut-off point of platelet reactivity in a stepwise or linear manner, and recent data have shown that a potential threshold of platelet reactivity, measured by ADP-induced LTA or the POC VerifyNow assay, may be associated with an increased risk of post-discharge ischemic events after PCI [22-25,29-31]. This suggests that in patients with platelet reactivity values below a threshold point, the risk of adverse clinical events is minimal.
An definite threshold of HPPR has not been established [32]. Bliden et al. [33] demonstrated that PCI-treaty patients with a 5 µmol/L ADP-induced Aggmax ≥ 50% were at increased risk for recurrent ischemic events (odds ratio, 34.6; 95% CI, 8.3 to 144.2; p < 0.001). Based on this suggested value, our patients demonstrated an increased risk of HPPR after switching from Plavix® to Plavitor® (0% vs. 30%, p < 0.001). However, when we adopted another suggested value of PRU ≥ 240 [29-31], switching from Plavix® to Plavitor® produced a reduced risk of high residual platelet reactivity (45% vs. 35%, p = 0.009). Although there were some differences between the results obtained with the two methods, poor inter-therapy agreement suggests that switching medications may increase the possibility of risk of adverse ischemic events in some patient. Therefore, physicians should be cautious when routinely switching to generic clopidogrel due to cost, particularly during the early phase of coronary stenting or ACS.
Recently, Kimura et al. [34] demonstrated that Japanese patients with sirolimus-eluting stents showed a relatively lower incidence of ST (0.2% per year), and discontinuation of thienopyridine only in these patients was not associated with any increased risk of ST beyond 6 months. Based on these data, we may consider that switching to generic clopidogrel may be possible in Asian patients be, and 6 months after coronary stenting. However, the decision to introduce generic clopidogrel might be determined based on analysis of platelet function, clinical risk factor, and lesion complexity.
The short duration of the study period and the small number of study subjects limits the conclusions that can be drawn from our study. Other limitation includes variability in LTA values with sample conditions and processing, even though an expert performed the platelet function tests and validation tests daily. However, while the present study does not have the statistical power to confirm the efficacy of Plavitor® over Plavix®, the pilot data justify conducting a larger, more definitive study.
In conclusion, among patients on Plavix® maintenance therapy after coronary stenting, a switch to Plavitor® produces a similar inhibition of ADP-induced platelet aggregation. However, inter-therapy agreement between platelet function measurements is relatively poor. Thus, physicians may be cautious when routinely introducing generic clopidogrel bisulfate.
Acknowledgements
This study was partly supported by grants from the Research Foundation of Gyeongsang National University Hospital, Jinju, Korea.
Notes
This study was partly supported by grants from the Dong-A Pharmaceutical Corporation, Seoul, Korea.
No potential conflict of interest relevant to this article was reported.