Long-Term Effects of Rosiglitazone on the Progressive Decline in Renal Function in Patients With Type 2 Diabetes
Article information
Abstract
Background/Aims
Thiazolidinediones reduce urinary albumin excretion and may prevent the development of renal injury. We evaluated the long-term effects of rosiglitazone on the progression of renal dysfunction in patients with type 2 diabetes mellitus.
Methods
We enrolled patients with type 2 diabetes mellitus who initially had normal or mildly impaired renal function, defined as an estimated glomerular filtration rate (eGFR) of 60-120 mL/min per 1.73 m2, and normoalbuminuria. Patients were divided into two groups according to their use of rosiglitazone during 3 years of follow-up: those treated with rosiglitazone (rosiglitazone group, n=52) and those treated without rosiglitazone (control group, n=85). Progression of renal dysfunction was defined as a decrease in eGFR of ≥9 mL/min per 1.73 m2 after 3 years.
Results
A greater difference was observed in the decrease in eGFR between the rosiglitazone and control groups after 3 years (3.8±9.9 vs. 12.6±10.5 mL/min per 1.73 m2, p<0.001). Seventeen of 52 (32.7%) patients in the rosiglitazone group and 53 of 85 (62.3%) patients in the control group showed progression of renal dysfunction (p=0.001). The progressors had a longer duration of diabetes (6.7±5.9 vs. 3.9±4.1 years, p=0.002), higher HbA1c levels (7.4±1.8 vs. 6.8±1.3%, p=0.023), and less frequent use of rosiglitazone (24.2 vs. 52.2%, p<0.001) compared to non-progressors. Multiple logistic regression analysis revealed that the use of rosiglitazone was a significant and independent predictor of the progression of renal dysfunction.
Conclusions
This study suggests that rosiglitazone theatment slows the progressive deterioration of renal function in patients with type 2 diabetes.
INTRODUCTION
The thiazolidinediones (TZDs), such as rosiglitazone and pioglitazone, comprise a class of agents that lower blood glucose through a reduction in insulin resistance in patients with type 2 diabetes. Apart from improving glycemic control, several experimental and clinical studies support the idea that TZDs also have beneficial effects on the progression of diabetic nephropathy [1-11]. The long-term effects of TZDs on renal dysfunction are important because patients often use them as continuous therapy for a long time. However, most studies on the renoprotective effects of TZDs in humans have been short term (<1 year), and the effects are typically only assessed by amelioration of albuminuria, not changes in renal function.
The American Diabetes Association recommends that serum creatinine and urinary albumin excretion (UAE) be measured at least annually in all patients with type 2 diabetes [12]. A substantial percentage of adults with diabetes have impaired renal function, defined as a glomerular filtration rate (GFR) <60 mL/minutes per 1.73 m2, but they also typically show normoalbuminuria [13,14]. In addition, only 33% of patients with diabetes who had GFR values of 30-60 mL/minutes per 1.73 m2 had serum creatinine values >120 µmol/L, the upper limit of normal [15]. This indicates that serum creatinine alone should not be used as a measure of kidney function, and that instead, esti-mated GFR should be used, regardless of the degree of UAE [15].
In the present study, we investigated the long-term effects of rosiglitazone on renal function in patients with type 2 diabetes who showed normoalbuminuria and normal or slightly decreased GFR.
METHODS
We conducted a retrospective study on patients with type 2 diabetes who visited the diabetes clinic at St. Mary's Hospital, Seoul, Korea, between October 2001 and September 2004 by reviewing their medical records. Only patients with a UAE <20 µg/minutes and a GFR of 60-120 mL/minutes per 1.73 m2 at baseline were included. We excluded patients with acute febrile infections, hematuria, pyuria, or a positive urine culture, and those who had been treated with rosiglitazone for less than 3 years. In total, 137 patients met the criteria. Patients were divided into two groups according to their use of rosiglitazone for 3 years: those treated with rosiglitazone (rosiglitazone group, n=52) and those treated without rosiglitazone (control group, n=85). Serum creatinine levels and UAE were measured every year.
After an overnight fast, blood samples were obtained for analysis of serum concentrations of creatinine, total cholesterol, triglyceride, and HDL cholesterol. Total cholesterol and triglyceride concentrations were measured enzymatically. HDL cholesterol concentrations were measured enzymatically after precipitation of the other lipoproteins. The HbA1c level was determined by high-performance liquid chromatography, with a reference range of 4.4-6.4%. UAE was measured by 24-hours urine collection. Albumin concentrations were deter-mined by immunoturbidimetry (Alb-Tia Seiken; Denka Seiken, Tokyo, Japan).
GFR was calculated using the Modification of Diet in Renal Disease (MDRD) formula:
estimated GFR (eGFR)=186.3×[SCr×0.011]-1.154×age-0.203 [×0.742, if female],
where SCr is serum creatinine expressed in µmol/L. Because of concerns about the validity of the MDRD equation for subjects with higher levels of kidney function and glomerular hyperfiltration in patients with early diabetes, subjects with baseline GFR values exceeding 120 mL/minutes per 1.73 m2 were also excluded [16]. Progression of renal dysfunction was defined as a decrease in the eGFR of ≥9 mL/minutes per 1.73 m2 after 3 years, as proposed by Klein et al. [17]. Renal function was graded according to the Kidney Disease Outcomes Quality Initiative guidelines [18]: stage 1, ≥90; stage 2, 60-89; stage 3, 30-59; stage 4, 15-29; and stage 5, <15 mL/min per 1.73 m2.
Statistical analyses were performed using the SPSS statistical package (SPSS Inc., Chicago, IL, USA). Because triglyceride values are not normally distributed, the data were analyzed after logarithmic transformation. Differences in continuous variables between the groups were analyzed by Student's t-test. The χ2 test was used to compare frequencies between the two groups. The correlation between the decrease of eGFR after 3 years and other variables in the study subjects was examined by Pearson correlation analysis. Multiple logistic regression was performed to assess the independent predictive effect of the variables on the risk for progression of renal dysfunction. P values <0.05 were deemed to be statistically significant.
RESULTS
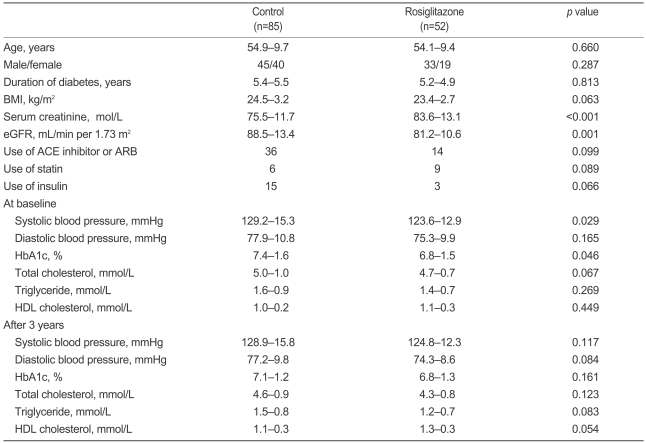
The clinical characteristics of the study subjects at baseline are summarized in Table 1. The control and rosiglitazone groups showed no significant difference in age, gender, BMI, duration of diabetes, use of an ACE inhibitor or an angiotensin II receptor blocker (ARB), statin use, or insulin use. Systolic blood pressure and HbA1c were lower in the rosiglitazone group than in the control group. The rosiglitazone group had higher serum creatinine levels and lower eGFR values than the control group. After 3 years, no significant difference in blood pressure, HbA1c, or lipid profiles was observed between the control and rosiglitazone groups (Table 1).
The median decrease in eGFR after 3 years in all subjects was 9.8 mL/minutes per 1.73 m2. The decrease in eGFR was not different between the two groups after 1 year (0.0±9.7 vs 2.8±11.4 mL/minutes per 1.73 m2, p=0.167). However, the decrease in eGFR was significantly less in the rosiglitazone group than in the control group after 2 years (2.5±9.5 vs. 6.9±11.4 mL/minutes per 1.73 m2, p=0.046) and 3 years (3.8 ±9.9 vs 12.6±10.5 mL/minutes per 1.73 m2, p<0.001, Fig. 1). As a result, the eGFR was similar after 3 years between the two groups (75.8±13.6 vs 76.8±11.1 mL/minutes per 1.73 m2, p=0.655), although it was higher at baseline in the control group than in the rosiglitazone group (88.5±13.4 vs 81.2±10.6 mL/minutes per 1.73 m2, p<0.001).

Comparison of the decrease in eGFR between control and rosiglitazone group during the follow-up period. The empty and closed circles represent the control group and rosiglitazone group, respectively. A bar indicates the mean value. *p<0.001 vs. the control group. eGFR, estimated glomerular filtration rate.
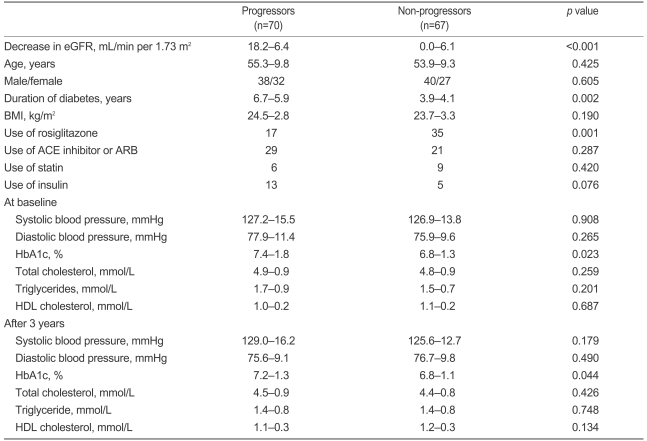
Seventy subjects experienced a progression in renal dysfunction ('progressors') after 3 years. Clinical and biochemical characteristics of progressors and non-progressors at baseline and after 3 years are shown in Table 2. The two groups did not differ significantly in age, gender, BMI, use of ACE inhibitors or ARBs, blood pressure, or lipid profiles. The progressors had a longer duration of diabetes (6.7±5.9 vs. 3.9±4.1 years, p=0.002), higher HbA1c levels (7.4±1.8 vs. 6.8±1.3%, p=0.023 at baseline; 7.2±1.3 vs. 6.8±1.1%, p=0.044 after 3 years), and less frequent use of rosiglitazone (24.2 vs. 52.2%, p<0.001) compared to the non-progressors. More importantly, 17 of 52 (32.7%) patients in the rosiglitazone group and 53 of 85 (62.3%) patients in the control group showed progression of renal dysfunction, as defined by a decrease in eGFR ≥9 mL/minutes per 1.73 m2 (p=0.001).
During the study period, 46 of 137 subjects (33.5%) showed deterioration to a more advanced stage of renal impairment, as defined by the Kidney Disease Outcomes Quality Initiative guidelines [18]. Ten of 52 patients deteriorated to a more advanced stage of renal impairment in the rosiglitazone group compared to 36 of 85 patients in the control group (19.2 vs. 42.3%, p=0.006). Additionally, 18 patients showed progression to microalbuminuria (a UAE of 20-200 µg/minutes): 3 of 52 patients in the rosiglitazone group and 15 of 85 patients in the control group (5.7 vs. 17.6%, p=0.046).
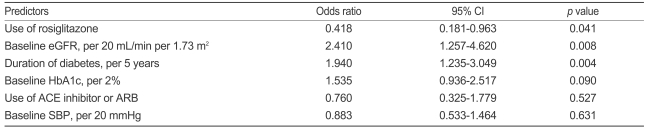
Multiple logistic regression analysis was used to identify independent predictors of the progression of renal function. Use of rosiglitazone, baseline eGFR, and duration of diabetes were all significant and independent predictors of the progression of renal dysfunction (Table 3).
DISCUSSION
This 3-year-old longitudinal study provides the first evidence that rosiglitazone can slow the progressive decline in renal function in patients having type 2 diabetes with normoalbuminuria and normal or slightly decreased GFR. This association was independent of initial renal function, ACE inhibition, glycemic control, or duration of diabetes.
Many studies have suggested that TZDs reduce urinary albumin or protein excretion and protect against injury to the kidney. Studies in animal models [1,2] suggest that TZDs can prevent diabetic nephropathy, and reduction in microalbuminuria has been demonstrated in patients having type 2 diabetes with all TZDs (troglitazone, pioglitazone, rosiglitazone) examined [3-11]. However, most of the studies on the renal effects of rosiglitazone have been limited by the short duration of follow-up, typically terminated within 12 months of treatment onset. Bakris et al. [4] examined the effects of rosiglitazone on UAE in a 52 weeks, open-label, cardiac safety study comparing rosiglitazone and glyburide. After 28 weeks of treatment, both groups had significant reductions (about 30%) in UAE, but after 52 weeks, only the rosiglitazone group continued to demonstrate a significant reduction in UAE. Thus, the duration of follow-up is an important variable in assessing the effects of rosiglitazone. In particular, the long-term effects of rosiglitazone on renal dysfunction are of interest because patients often use the drug as a continuous therapy. To our knowledge, however, no published study has examined the effects of TZDs on the progression of kidney disease. Thus, we sought to analyze the effects of rosiglitazone on long-term changes in renal function, assessed by eGFR, not UAE, in patients having type 2 diabetes with normoalbuminuria and normal or slightly decreased GFR.
First, we demonstrated that the rosiglitazone group showed slower deterioration of renal function, as assessed by the decrease in eGFR, over 3 years (3.8 mL/minutes per 1.73 m2 in the rosiglitazone group vs. 12.6 mL/minutes per 1.73 m2 in the control group). Second, fewer patients in the rosiglitazone group developed microalbuminuria during the follow-up period (5.7% in the rosiglitazone group vs. 17.6% in the control group), despite similar numbers of patients receiving ACE inhibitors or ARBs in each group. Third, 42.3% of the patients in the control group transitioned to a more advanced stage of renal dysfunction compared to 19.2% of those in the rosiglitazone group. Finally, the progressors who had a decrease in estimated GFR ≥9 mL/minutes per 1.73 m2 over 3 years had a longer duration of diabetes, higher HbA1c levels, and less use of rosiglitazone than the non-progressors. More than half of the non-progressors (35/67) were taking rosiglitazone. After adjusting for initial eGFR, duration of diabetes, HbA1c, and use of an ACE inhibitor/ARB, the use of rosiglitazone remained an independent predictor of renal outcome. Thus, the use of rosiglitazone was independently related to slowing the progression of renal dysfunction in our subjects, independent of initial renal function, ACE inhibition, hyperglycemia, and duration of diabetes. Because patients in the present study had normoalbuminuria, with normal or mildly decreased GFR, the possibility exists that the renoprotective effects of rosiglitazone could have occurred at earlier stages of kidney disease.
The mechanisms through which TZDs reduce UAE and renal injury are unclear [19]. Several systemic actions of TZDs, such as reductions in blood glucose and insulin, as well as BP levels, could be involved in the renoprotective action of these compounds. TZDs initiate their action by binding to peroxisome proliferators-activated receptor-γ (PPARγ). Although PPARγ receptors are most abundant in fat cells, they have also been demonstrated in renal mesangium and glomerulus. Transforming growth factor-β (TGF-β) has been recognized as a central player in the fibrogenic process of diabetic nephropathy, through both stimulatory effects on matrix production and blocking of matrix degradation [20]. TZDs suppress the renal expression of TGF-β reduce urinary albumin excretion, and ameliorate glomerular injury [21,22]. Miyazaki et al. [7] reported that decreased albuminuria by rosiglitazone treatment in patients with type 2 diabetes was independently associated with a decrease in TNF-α and an increase in adiponectin. Thus, TZDs may exert their renoprotective actions either directly by acting on renal PPARγ receptors or indirectly by altering adipocytokines released from fat cells.
This study was limited by its retrospective design. Patients in the rosiglitazone group had significantly lower systolic blood pressures and HbA1c values at the start of followup. Because these variables are key predictors of the development and progression of diabetic nephropathy, we attempted to correct for this confounding factor using multiple regression analysis. The study was also limited by the imperfect method of estimating renal function. The MDRD equation may underestimate GFR in subjects with normal renal function, especially those with a GFR ≥90 mL/minutes per 1.73 m2 [2,23]. However, the primary interest in our study was in the changes in kidney function, and such concerns were deemed relatively unimportant.
In conclusion, our data suggest that TZDs slow the progressive deterioration of renal function in patients with type 2 diabetes. However, long-term prospective studies are required to confirm the findings, especially in patients with overt proteinuria or renal insufficiency.