Castleman Disease Presenting with Jaundice: A Case with the Multicentric Hyaline Vascular Variant
Article information
Abstract
Castleman disease (CD) is a rare lymphoproliferative disorder of unknown etiology with different clinical manifestations. A previous healthy 50 year-old man was hospitalized for right upper quadrant (RUQ) abdominal pain. He had jaundice and a 1 cm-sized lymph node in the right supraclavicular area. Pancreas and biliary computed tomography (CT) showed masses at the right renal hilum and peripancreatic areas. Positron emission tomography (PET) showed widespread systemic lymphadenopathy. Excisional biopsy of the right supraclavicular node revealed a hyaline vascular variant of CD. Corticosteroid therapy was started and the extent of disease decreased. We here report a case of multicentric CD, the hyaline vascular variant, presenting with jaundice, diagnosed by excisional biopsy and successfully treated with corticosteroids.
INTRODUCTION
Castleman disease (CD) is a rare lymphoproliferative disorder. Clinical features of the disease vary from asymptomatic to symptomatic lymphadenopathy accompanied by fever, malaise and/or weight loss. Based on the histological findings CD is divided into two variants the hyaline vascular variant (HVV) and the plasma cell variant (PCV). When disease location is considered, CD can be divided further into three types including a unicentric HVV found in 72% of cases, unicentric PCV accounting for 18% of cases and multicentric PCV identified in 10%. The following case has two unique features: (1) presentation as an obstructive jaundice mimicking acute cholangitis and (2) presentation as a widespread systemic lymphadenopathy. The final diagnosis was confirmed as multicentric HVV, which is a very rare variant accounting for only 1% of all CD cases.
CASE REPORT
A previously healthy 50 year-old male patient visited the emergency department for right upper quadrant abdominal pain. He had a smoking history of 20 pack-years and quit smoking seven years ago. He had no history of liver disease or other illness except prurigo simplex and papular urticaria on both lower extremities. Three weeks prior to presentation, he visited a local clinic for loss of appetite and weight loss (4 kg/3 months). Gastrofibroscopy and colonoscopy findings were normal. One week prior to presentation, the patient began to have RUQ discomfort, which progressed to pain in three days. Physical findings were normal except for a yellowish skin color and a 1 cm-sized lymph node at the right supraclavicular area. Initial laboratory testing showed: total bilirubin 6.0 mg/dL, ALP 280 IU/L, AST/ALT 125/248 IU/L, gamma-GT 195 IU/L, lipase 1648 U/L (23~300), and amylase 140 U/L (60~180). The complete blood cell count (CBC) was normal except for a slightly increased eosinophil count (668/µL). Serum antinuclear antibody, rheumatoid factor and anti-HIV antibodies were all negative. Pancreas and biliary CT showed masses at the right renal hilum and the peripancreatic area (Figure 1). Percutaneous needle biopsy (PCNB) from the renal hilum revealed a patchy infiltration of atypical lymphoplasma cells. However, lack of sufficient tissue limited an accurate diagnosis. Evaluation for additional lymphadenopathy with whole body PET was done. It revealed multiple positive uptakes in the cervical, right hilar, subcarinal, and subaortic lymph nodes as well as in the peripancreatic area (Figure 2). Additional neck and chest CT showed multiple enlarged homogeneously enhancing lymph nodes on both side of neck and mediastinum. Excisional biopsy of the right supraclavicular node revealed a hyaline vascular variant of CD (Figure 3). Immunohistochemical findings were CD20 (+), CD3 (+), CD21 (+), CD10 (+ in germinal center), bcl-2 (+ in paracortical area), and HHV-8(-). HHV-8 PCR from peripheral blood mononuclear cells was also negative.
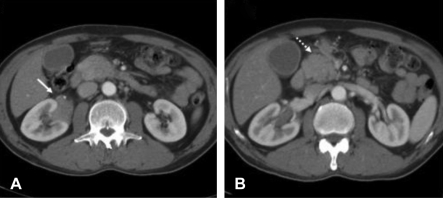
(A) Contrast-enhanced CT scan shows a homogeneous mass (arrow) infiltrating the right renal sinus. This mass does not result in vascular occlusion or hydronephrosis. (B) Another homogeneously enhancing soft tissue mass (dotted arrow) is noted in the peripancreatic area.

FDG PET images were obtained 60 min after intravenous injection of 370 MBq of [18F]-FDG using an Allegro PET scanner (Philips Medical System, Cleveland, OH., USA). 2.5 minutes emission scans, with the nine-table position, from the level of the proximal femur to the cerebellum obtained using the 3D acquisition mode. An attenuation map obtained using a Cs-137 transmission source. Attenuation correction images reconstructed using a 3D-RAMLA algorithm with a 3D image filter. (A-D) Coronal PET images show [18F]FDG-avid hyperplastic lymph nodes at bilateral cervical areas, mediastinum, hilar area, mid abdomen, and renal hilar area. (E~H) Transaxial PET images co-registered with enhanced CT. Arrows indicate FDG accumulation at the renal hilar lesion (arrow) and the pancreatic body (dotted arrow)
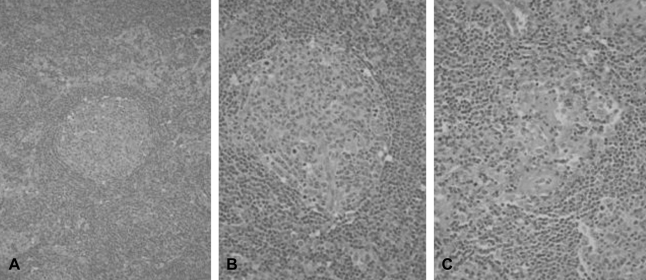
(A) Concentric layers of small lymphocytes seen around the reactive germinal centers (original magnification ×200; hematoxylin-eosin stain). (B) A lymphoid follicle with a partially involved germinal center is transfixed by a penetrating arteriole. Concentric layers of small uniform lymphocytes result in an 'onion-skin' appearance (original magnification ×400; hematoxylin-eosin stain). (C) Blood vessels between the follicles are lined by hyperplastic endothelial cells and surrounded by fibrocollagen (original magnification ×400; hematoxylin-eosin stain).
After the administration of high dose corticosteroids (1 mg/kg of prednisolone), liver function improved with the level of total bilirubin decreasing from 16.3 to 2.1 mg/dL, ALP from 348 to 110 IU/dL and AST/ALT from 291/1054 to 29/122 IU/L. After two weeks of treatment, follow-up abdominal CT showed that pancreatic swelling, the pararenal mass and the multiple peripancreatic lymph nodes decreased in size (Figure 4).
DISCUSSION
Castleman disease is rare, and its scarcity has precluded comprehensive studies leading to an incomplete understanding of its pathophysiology1). Recent reports suggest that HHV-8 infection stimulates B lymphocytes to induce lL-6 production in the mantle zone2-4). The proportion of HHV-8 infected cases among CD patients is variable and reported to be from 33% to 100%. In HIV-positive patients, nearly all CD cases have been positive for HHV-85). In our case, immunohistochemical staining of lymph nodes and peripheral blood PCR revealed no correlation between HHV-8 infection and the disease. Other exogenous or endogenous factors may induce IL-6 secretion from B lymphocytes in HHV-8 negative CD. Local production of IL-6 may contribute to the characteristic B-cell proliferation and vascularization observed in CD6). Moreover, in patients with multicentric CD, systemic symptoms might result from the circulation of IL-6 and IL-6-producing B lymphocytes7).
In order to diagnose CD, exclusion of other diseases with lymphadenopathy is very important. These include rheumatoid arthritis, lupus, Sjogren syndrome, HIV infection, lymphoma, and drug sensitivity. For our patient we initially thought he had lymphoma causing an obstructive jaundice and rapid deterioration. There were no specific symptoms, signs or history suggestive of inflammation, infection or drug sensitivity. We used the PCNB of the perirenal mass as a diagnostic tool initially in order to start chemotherapy immediately. Since we failed to get enough tissue from the PCNB of the perirenal mass, we next tried an excisional biopsy of the cervical node.
There are three different presentations for CD. The most common type is the unicentric hyaline vascular variant (U-HVV) accounting for 72% of all CDs. A single node or a chain of lymph nodes is involved in the U-HVV variant, and its most common location is the mediastinum. The unicentric plasma cell variant (U-PCV) accounts for 18% of all cases of CD; most U-PCV patients have constitutional symptoms such as anemia, elevated ESR and frequently abdominal lymph nodes. The final type is the multicentric plasma cell variant (M-PCV) representing 10% of all CDs. The M-PCV patients are usually older than in the other variants commonly in the fifth to sixth decade of life. Patients with M-PCV frequently present with fever, organomegaly and/or abnormal laboratory findings such as anemia or thrombocytopenia9).
Our case presented with an abdominal mass and systemic lymphadenopathy. The histological type was confirmed HVV. Neither fever nor organomegaly were present in this patient. The only symptoms noted on presentation were those commonly associated with an obstructive jaundice, anorexia and/or weight loss. The proportion of patients with muticentric CD and HVV is less than 1% of all CD. Soulier J reported four patients with HVV among 31 patients with multicentric CD10). Two of these patients had systemic symptoms and signs and one patient had HIV infection and Kaposi's sarcoma. Two patients were male and three patients were positive for HHV-8 by PCR.
We could find only two prior reported cases of CD with obstructive jaundice11, 12). A filiform stricture of the common hepatic duct was noted in the first case. Laparotomy confirmed U-HVV involving the bile ducts, liver parenchyma and adjacent lymph nodes. The second case presented with enlarged lymph nodes in the porta hepatis and the common bile duct. This case was confirmed as the HVV variant of CD by laparotomy.
The therapeutic approach to multicentric CD depends on whether HHV-8 PCR and CD20 are positive. In HHV-8 PCR-positive cases, use of valgancilovir is considered. For the CD20-positive cases, treatment is with rituximab an anti-CD20 monoclonal antibody5). Patients presenting with both markers negative receive treatment with immunosuppressive agents such as prednisolone, interferon-alpha, thalidomide, or CHOP chemotherapy. Our patient was HHV-8 negative and CD20 positive. The use of prednisolone resulted in an immediate decrease in the size of the mass and improved laboratory profiles. Follow up evaluations of symptoms, laboratory tests including liver function and image studies at regular intervals are planned for this patient.
In conclusion, we present a case of multicentric CD presenting with jaundice. PET identified widespread systemic lymphadenopathy; the final diagnosis was confirmed as CD, the hyaline vascular variant.
