A Case of McCune-Albright Syndrome with Associated Multiple Endocrinopathies
Article information
Abstract
McCune-Albright syndrome (MAS) is a rare disorder that develops from an activating mutation in the Gs gene. It is characterized by an association with Polyostotic fibrous dysplasia, and precocious puberty, Caf-au-lait pigmentation, and other endocrinopathies that result from the hyperactivity of a variety of endocrine glands. Recently we encountered a patient with MAS with fibrous dysplasia, skin pigmentation, acromegaly, hyperprolactinemia and a thyroid nodule. A 23-year-old male presented for an evaluation of a change in his facial structures. Fibrous dysplasia was diagnosed by a bone biopsy and radiographic studies. The GH level increased paradoxically after an oral glucose load. The plasma prolactin, IGF-1 and alkaline phosphatase were high. Thyroid ultrasonography revealed multiple nodules. The brain MRI demonstrated a mass in the left pituitary gland. Genetic analysis identified a change from Arg (CGT) at codon 201 to Cys (TGT).
INTRODUCTION
McCune-Albright syndrome (MAS) is a rare disease that was originally defined by the triad of polyostotic fibrous dysplasia of the bone (FD), caf-au-lait skin pigmentation and precocious puberty1, 2). A number of endocrine disorders such as hyperthyroidism, excess growth hormone (GH), Cushing syndrome3) and rickets/osteomalacia might be associated with MAS3, 4). The disease results from post zygotic activating mutations of the cAMP regulating protein, GNAS1 gene product, and Gs5, 6). Most Gs mutations are point mutations at the Arg201 position, with the majority being Arg201 His or Cys7). Recently, weevaluated a 23-year-old man with typical MAS who had FD, skin pigmentation and multiple endocrinopathies including acromegaly, hyperprolactinemia and a thyroid nodule. Genetic analysis confirmed a point mutation of the GNAS1 gene. We report this case with a review of the relevant literature.
CASE REPORT
A 23 year-old man was referred to our hospital for an evaluation of a change in his facial structures that started from the age of 17 years. His facial features gradually coarsened, and his nose was deviated to the right side. He experienced a progressive increase in height with the growth of his hands and feet, and had thick fingers and toes for the last six to seven years. He was recently diagnosed at a local clinic with diabetes mellitus and had been treated with oral glimepiride (1 mg/day). In addition, he had visual problems that were diagnosed when he was eight as well as FD diagnosed by a bone biopsy in another hospital. He had undergone maxillary decompression surgery and had a history of femur fractures after trauma. His father died at a young age and his mother had diabetes mellitus and visual loss.
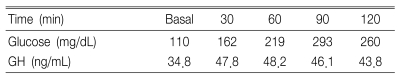
A physical examination revealed that his height, weight and body mass index were 185 cm, 87 kg and 27.0 kg/m2, respectively. The BP was 130/80 mmHg. He had mandibular prognathism, maxillomandibular malocclusion, and acral enlargement with caf-au-lait pigmentation on the back and lips (Figure 1A, 1B). There were no abnormalities in his external genitalia. The results of the complete blood count, liver and renal function test were all normal. The fasting and two-hour whole-blood glucose levels were 162 and 293 mg/dL, respectively. The serum calcium and phosphate levels were 9 mg/dL (8.6-10.3) and 2.9 mg/dL (2.5-4.5), respectively. The iPTH level, alkaline phosphatase level, serum osteocalcin level and urine deoxypyridinoline/Creatinine ratio was 61.4 pg/mL (12-72), 950 IU/L (75-270), 288.10 ng/mL (10.3-23.7) and 37.3 nM/mM (2.5-5.5), respectively. The basal GH level was 34.8 ng/mL, which paradoxically increased to 48.2 ng/mL (N<2 ng/mL) after a 75 gm oral glucose load (Table 1).
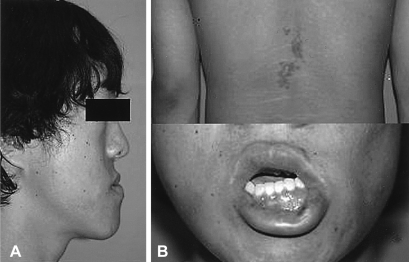
General appearance of the 23-year-old patient. The aptient had mandibular prognathism (A), and an irregular shaped, but not elevated black and brown colored pigmentation on the back and lips (B).
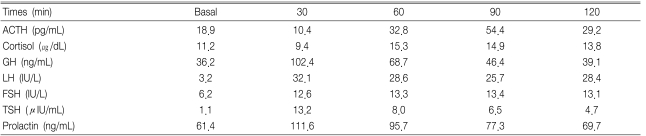
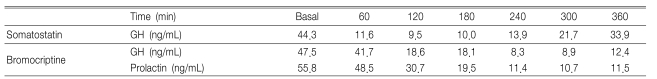
In response to a 400 µg IV bolus thyrotropin releasing hormone (TRH), the GH level increased from 36.2 ng/mL to 68.7 ng/mL (Table 2), and the serum IGF-1 level was 1544.2 ng/mL (100-366). The serum prolactin level was 61.4 ng/mL (2.1-17.7) and the total testosterone level was 1.1 ng/mL (2.4-8.1). The level of TSH, total T3 and free T4 were 3.0 IU/mL (0.4-4.7), 1.66 ng/mL (0.6-1.7) and 1.1 ng/mL (0.7-1.9), respectively. The basal ACTH and cortisol levels were normal but the response of ACTH and cortisol was diminished on the combined pituitary function test (Table 2). The level of GH secretion decreased when he was administered somatostatin and bromocriptine, (Table 3).
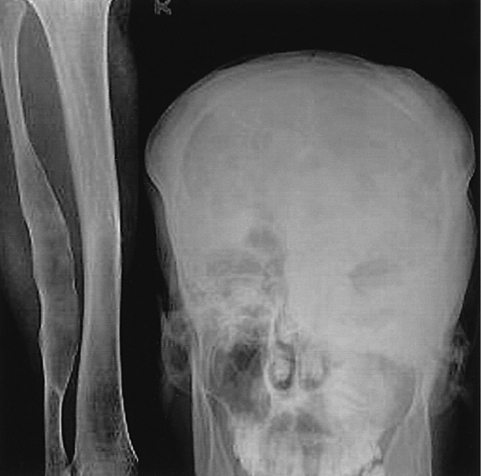
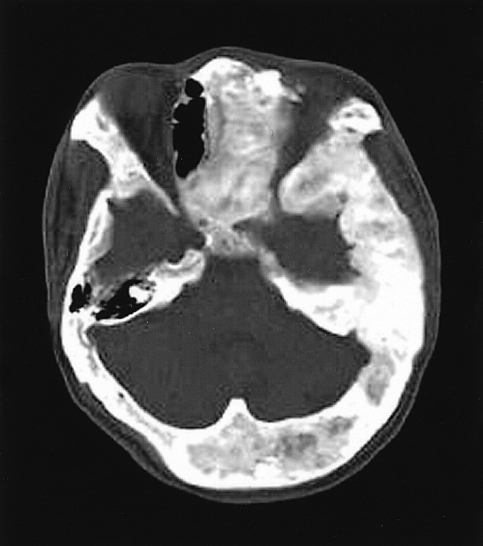
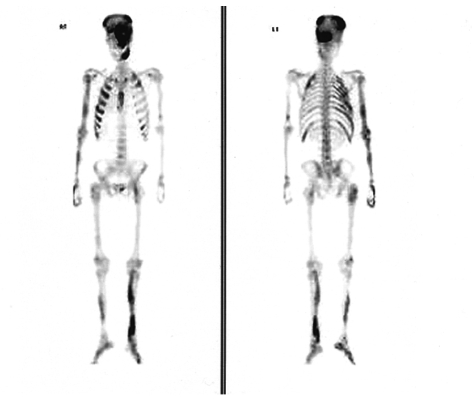
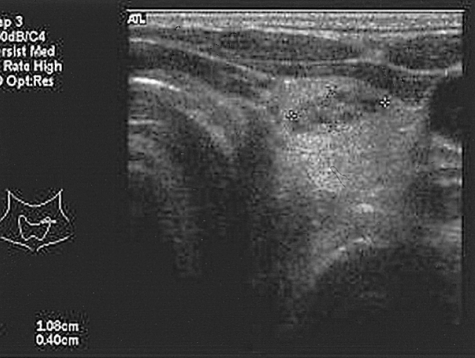
The X-ray revealed that the skull and facial bones had a wavy thickening and deformity. There was a well-defined osteolytic mass in the left humerus, the distal portion, as well as ground-glass opacity inside of the bone. In addition, a similar osteolytic lesion was observed in the right foot, both femurs, right fibula, right iliac bone and left scapula. In addition, there was diffuse thickening and a deformity of the ribs (Figure 2). The heel pad index was 24.7 mm. The Sellar MRI of the brain revealed an infiltrative solid mass (13×8 mm) in the left pituitary gland extending into the left cavernous sinus with encasement of the left carotid artery (Figure 3). Contrast enhanced computerized tomography of brain revealed lesions with bony thickening and a deformity at the skull base (Figure 4). The bone scan showed multiple hot spots with uptake at the skull, facial bone, ribs, humerus and tibia (Figure 5). Thyroid ultrasonography indicated multiple ill defined solid hypoechoic nodules in both lobes of the thyroid (Figure 6). The result of a fine needle aspiration was adenomatous hyperplasia. Esophagoduodenoscopy and colonoscopy did not reveal any abnormalities. Genetic analysis for a mutationwas performed by sequencing the polymerase chain reaction (PCR)-amplified genomic DNA. DNA was extracted from the venous blood, pigmented skin and a thyroid nodule. Base sequencing was carried out using the sense (5'-TGACTATGTGCCGAGCGA-3') and antisense primers (5'-CCACGTCAAACATGCTGGTG-3'). The mutation was identified only in the thyroid tissue. There was a change from Arg (CGT) in codon 201 to Cys (TGT) at the R201C position (Figure 7A, 7B).
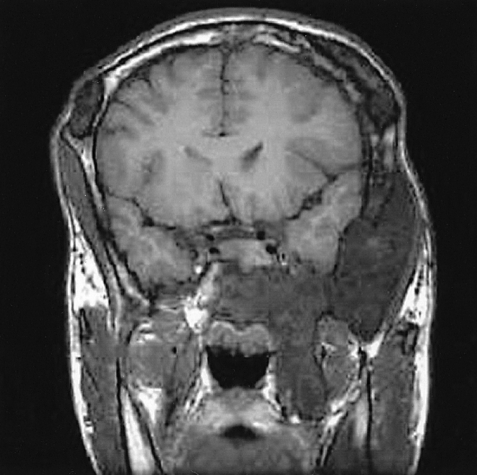
Coronal image of a sellar MRI of the brain shows an infiltrative solid mass in the left pituitary gland with a cavernous sinus extension encasing the carotid artery.

Mutational analysis of the genomic DNA isolated from the thyroid tissue using standard PCR amplification and direct sequencing. Forward sequencing reaction of the genomic DNA shows a clear red T peak in the wild-type C due to C-to-T mutation at R201C transition in the GNSA1 gene. (B) Reverse sequencing reaction of the genomic DNA also shows an A peak under G.
The percentage of cells with a mutation in GNAS1 out of the total number of cells from each tissue was so low that it was difficult to identify by direct base sequencing. Therefore, mutation allele specific amplification was carried out. PCR was completed after combining the sense primer (5'-CCTGCTTCGCTGCC-3'), which did not have a mutation and was considered to be the wild type, and the sense primer (5'-CCTGCTTCGCTGCT -3'), which was the mutation type with the antisense primer (5'-GGACTGGGGTCAATGTCAAG-3'), separately. As a result of the mutation allele specific amplification, the mutant type allele was detected in the blood, pigmented skin and thyroid tissue from the affected patient (Figure 8). The skull deformity was too severe from FD to perform surgery. Therefore, medical treatment with somatostain (sandostatin LAR 30 mg IM q month) and bromocriptine (Antilactin 7.5 mg bid PO) was started. The prolactin level was kept within normal limits, and the GH and IGF-1 levels had a tendency to decrease (GH 5.9 ng/mL, IGF-1 786 ng/mL). The FD of the bone is under observation and Pamidronate treatment is being considered.
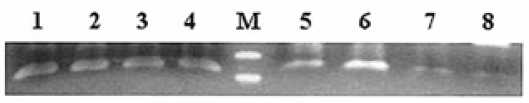
Determination of a GNSA1 mutation using mutant allele specific amplification shows that the mutant type allele 1 was detected in all specimens from the patient including: blood, pigmented skin and thyroid tissue. 1 and 5, 2 and 6, 3 and 7, 4 and 8 : PCR reactions with genomic DNA from blood, skin and thyroid tissue from the patient and a negative control, respectively1,2,3,4 : wild type primer, 5,6,7,8 : mutant type primer
DISCUSSION
Typical MAS is characterized by FD with at least one of the typical hyperfunctioning endocrinopathies along with caf-au-lait spots. Unlike a typical case of MAS, patients with FD and more than two endocrine disorders without skin pigmentation or precocious puberty are defined as having atypical MAS8). Although typical MAS is relatively rare, FD of the bone not associated with either skin pigmentation or endocrine disturbances is a frequent and usually serious skeletal disorder9).
Only three cases of typical or atypical MAS have been reported in Korea10-12). Bony fibrous dysplasia most commonly occurs in the proximal femur and at the base of the skull. FD of the skeleton usually presents with a limp or pain but occasionally a pathologic fracture may be the presenting sign. FD of the craniofacial bones can cause visual disturbances, proptosis, nasal obstructions, facial asymmetry or hearing abnormalities.
The endocrine disorders accompanying MAS are precocious puberty, pituitary adenomas secreting growth hormone and/or prolactin, Cushing syndrome, hypophosphatemic osteomalacia, thyroid abnormalities (hyperthyroidism and benign and malignant thyroid nodule) and hyperparathyroidism3, 4, 13). Growth hormone hypersecretion in MAS is common but differs from that observed in classic acromegaly in several aspects. Patients are generally young at onset and are diagnosed based on the observation of growth acceleration rather than from the facial dysmorphism, which is usually difficult to assess due to fibrous dysplasia14). MAS with a GH excess has a distinct clinical phenotype that is characterized by the TRH responsiveness, prolactin co-secretion, small or absent pituitary tumors, a consistent but inadequate response to a treatment with cabergoline, and an intermediate response to sandostatin LAR. Vision and hearing deficits are more common in MAS with an excess of GH than in those without an excess. Patients with precocious puberty and an excess of GH have achieved a normal adult height despite a history of early epiphyseal fusion.
MAS syndrome is the result of an activating mutation of the alpha subunit of the Gs protein (GNAS1 gene), which causes the constitutive activation of adenylate cyclase. In all published cases of MAS, mutations of GS at the R201 position have been identified9). The unproven but accepted explanation for the lack of vertical transmission, along with the observation that the skin and bone lesions tend to lateralize, is that the disease results from postzygotic mutations. Hence, the patients are referred to as somatic mosaics. The mosaicism could explain the varied clinical picture of those with this syndrome5). The percentage of cells with the GNAS1 mutation out of a total number of cells in each tissue might be very low due to the mosaicism. This explains why GS mutations at the R201 position are detected only in the thyroid tissue by direct base sequencing, as in our case. However, the detection of a mutation in all samples was obtained after using the mutation allele specific amplification method. FD presents a treatment dilemma because there is no proven medical therapy. Bisphosphates have been shown to reduce the incidence of fractures and reduce the intensity of bone pain. However, the only cure for FD is a surgical resection, which is difficult in the skull and facial regions. Surgery is recommended when there is a functional cranial nerve deficit in order to prevent or limit the permanent loss of function15). Medical treatment using a somatostatin or dopamine agonist is often the only option in patients with MAS and an excess of GH because transsphenoidal surgery is not possible due to the massive thickening of the skull base with FD14, 16, 17). Radiation therapy is not considered to be an option because it is believed to predispose FD to a sarcomatous transformation18, 19). Hyperthyroidism in a MAS setting is treated with the same medication options used for treating hyperthyroidism4).
Wepresent a case of Typical MAS with FD, skin pigmentation, acromegaly, hyperprolactinemia, and a thyroid nodule in a 23-year-old man.