Benign Pulmonary Metastasizing Leiomyomatosis: Case Report and a Review of the Literature
Article information
Abstract
The authors report here on a case of a nearly asymptomatic 51-year-old Korean woman who was found to have diffuse, multiple nodules of the lungs on a routine chest radiograph. She had undergone hysterectomy 16 years previously for uterine myoma. An open lung biopsy revealed tumor that was composed of interlacing bundles of spindle cells with cigar shaped nucleus and eosinophilic myofibrils in the cytoplasm; consistent with multiple leiomyomas. The stains for SMA, desmin, MSA and Ki-67 were positive and the stain for c-kit was negative. The other stains for estrogen and progesterone receptor were positive. During the open lung biopsy procedure, all the nodules were excised.
We report here on an interesting case of benign metastasizing leiomyoma (BML) in 51-year-old patient. To the best of our knowledge, this case showed the longest period of clinical progression in Korea. This is also one of a few cases in which curative excision was successfully performed.
INTRODUCTION
Benign metastasizing leiomyomatosis (BML) is a rare entity and it previously has been thought to be part of a spectrum that also includes lymphangioleiomyomatosis because both diseases are hormonally influenced and remission has been reported following menopause or surgical castration. Total resection of the multiple nodules can only be performed in a very limited number of cases1, 2). These patients are usually asymptomatic and the clinical suspicion begins with the incidental discovery of pulmonary lesions.
We report here on an interesting case of BML in a 51-year-old patient who, to the best of our knowledge, is the oldest such patient described and who had the longest period of clinical follow-up in Korea. This is also one of a limited number of cases in which complete removal of the nodules was possible.
CASE REPORT
A 51-year-old Korean woman was referred to our pulmonary medicine outpatient clinic for the evaluation of her known multiple lung nodules: the nodules were first detected 4 months before the referral (Figure 1). However, the sizes of the nodules were very small at the time of discovery, reaching 0.7 cm diameter for the largest one on chest CT. The doctor, who was treating her for osteoporosis, maintained close observation and this included short term CT follow-up. The follow-up chest CT was done 4 months later just before the referral; it showed that the sizes of the masses were increased and multiple masses had been developed (Figure 2). She complained of mild, non-productive cough and general weakness of several weeks duration, but any other respiratory symptoms like hemoptysis, purulent sputum or exertional dyspnea were absent. She denied any fever, chill, weight loss, headache, myalgia or any other major symptoms. Her past medical history was remarkable in that she was previously diagnosed with uterine leiomyoma and underwent hysterectomy with right-sided oophorectomy approximately 16 years ago. She also underwent surgery due to acute appendicitis 27 years ago. She did not smoke and she rarely drank alcohol. She was previously diagnosed with osteoporosis a few years ago and was taking medication for it.
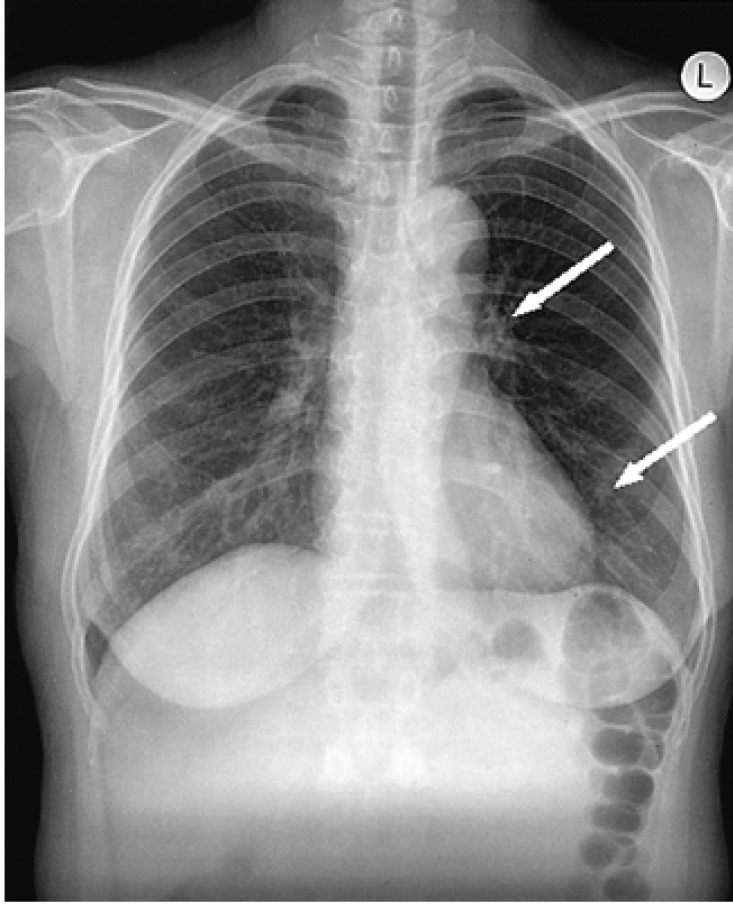
Initial chest radiograph shows two small nodular lesions (arrows) in left perihilar and retrocardiac areas.

Chest CT that was done 4 month later shows well-demarcated 0.7 cm sized two nodules (arrows) in the left upper lobe (Figure A) and left lower lobe (Figure B), and focal bronchopneumonia is seen in the right middle lobe.
The physical examination revealed that she was afebrile and appeared comfortable. There was no pallor, jaundice, cyanosis, or cold sweating. Her respiratory rate was 20 per minutes, the pulse rate was 90 per minute, the blood pressure was 140/90 mmHg and her body weight was 52 kg. Examination of the head, ears, eyes and throat was unremarkable. No lymphadenopathy or thyroid gland enlargement was noted. There was no neck vein distention. Auscultation of the lung revealed no definite crackle or wheeze. Examination of the heart revealed normal sounds without any gallops or murmurs. Examination of the abdomen and extremities was unremarkable without any palpable masses, hepatosplenomegaly or ascites. There were scars from a previous hysterectomy and previous appendectomy. The neurologic examination was normal. A 12-lead electrocardiogram revealed normal sinus rhythm. Laboratory studies including a complete blood count, a serum metabolic assay with liver function tests, the erythrocyte sedimentation rate, C-reactive protein, rheumatoid factor and anti-nuclear antibodies; all the tests showed normal results. Routine urine analysis for the cell count and casts, and the thyroid function test were also normal. Gynecologic examination revealed a normal hysterectomy status.
A chest radiograph taken at the time of first detecting the lung nodules demonstrated lung nodules that were especially scattered at the left perihilar and retrocardiac areas. The follow-up chest radiograph taken after admission showed newly developed peribronchial infiltration at the right middle lung field and the previously detected lung nodules had increased in size and number. Further evaluation led to a computed tomography scan of the chest, sputum studies and a tumor marker study; fine needle aspiration of the nodules was also performed. A computed tomography scan of the abdomen, a whole body bone scan, gastrofiberoscopy, sigmoidoscopy and breast sonography were also performed to search for the primary origin of suspicious metastatic lung nodules. Sputum specimens were all negative for bacterial, mycobacterial and fungal pathogens. The sputum cytology revealed no malignant cells. The serum tumor marker study, including alpha-feto protein, CEA, CA 19-9, CA 125, SCC and NSE, were all in the normal ranges. The computed tomography scan of the abdomen showed focal wall thickening in the right portion of rectum and a 4.1×3.2 cm-sized benign-looking cystic lesion in the left ovary. Yet the sigmoidoscopy revealed no abnormal findings. Gastrofiberoscopy revealed atrophic gastritis at the antrum and body of the stomach. The sample from the fine needle aspiration of the nodule was insufficient for diagnosis. Therefore, open lung biopsy was eventually performed. The pathologic findings from the open lung biopsy revealed a diagnosis of multiple leiomyomas of the lung. The tumor was composed of interlacing bundles of spindle cells with cigar shaped nucleus and eosinophilic myofibrils in the cytoplasm. The tumor was rather cellular, but there were no mitotic figures or cellular pleomorphism. The immunohistochemical staining results of the specimen were positive for SMA, desmin and MSA, all of which are smooth muscle antigens, and the specimen was also positive for Ki-67 (2%) (Figure 3). In addition to the positive results, it was negative for the c-kit, CD34, EBC-LMP, p53 and cytokeratin. We later performed staining for estrogen and progesterone receptors, and the result was positive for both receptors. We tried to compare these findings to the pathology of the previous uterine leiomyoma. Because of the long time interval between the patient's hysterectomy and open lung biopsy, her medical records and the pathologic specimen from her previous operation was lost. So, we could not compare each specimen. During the open lung biopsy procedure, all the visible nodules were excised.

Histologic findings of the nodules resected from the left lung. (A) Hematoxylin and Eosin stain (×100). (B) Immunohistochemical staining for smooth muscle actin was positive (×300). (C) Immunohistochemical staining for desmin was positive (×300).
Because any malignant potential was not completely ruled out in view of her previous history of uterine leiomyoma and the progressive enlargement on follow-up, we considered starting chemotherapy. Yet we decided on close observation instead of adjuvant chemotherapy due to the lack of evidence for achieving any benefit from chemotherapy for the metastasizing leiomyomatosis and also because side effects were possible. In addition, our previous experience and also the published reports have shown that benign metastasizing leiomyomatosis might regress spontaneously with menopause; we are now considering anti-hormonal therapy.
DISCUSSION
We observed a patient with benign metastasizing leiomyomatosis, and to the best of our knowledge, this is the 4th reported case in Korea3-5). In this case, complete excision of the multiple nodules was performed. The duration between the uterine and pulmonary phases of leiomyomata was 16 years, which is the longest of the 4 total cases reported in Korea. Our patient is also the oldest one in Korea.
We could not compare the pathologic specimens of the 2 surgeries, i.e., hysterectomy and open lung biopsy, but with the radiological characteristics of multiple lung nodules scattered in the bilateral lung fields, we concluded that the condition of this patient was benign metastasizing leiomyomatosis rather than primary pulmonary leiomyomatosis.
Benign metastasizing leiomyomatosis (BMLs) have also been called leiomyomatous hamartoma or fibroleiomyomatous hamartoma, and they are a rare disorder with about 75 documented cases in the literature until 20016). The patients in these reports have a history of histologically benign uterine leiomyoma and they may present several years later with multiple pulmonary, nodal or abdominal nodules that appear to be of smooth muscle origin; these nodules are most commonly seen in both lungs7).
BML has been described as originating from a uterine smooth muscle tumor of an unknown malignant potential, which, because of the limitations of histopathologic testing, cannot easily be classified as having malignant potential8). The tumor may actually represent a heterogeneous group of smooth muscles tumor, ranging from leiomyomas to low grade leiomyosarcomas8).
Most of the BML patients have undergone a hysterectomy 0 to 24 years earlier9). They are generally asymptomatic; therefore, the initial detection of the disease comes from other examinations for other purposes, like an annual health check examination. Yet there are a few cases presenting with symptoms such as dyspnea, dry cough, or chest pain9, 10).
The pathogenesis of the disease is unclear. Of the several possible mechanisms, hormone-dependent tumor growth might be the most popularly accepted as spontaneous regression of the disease in pregnancy11) and during the menopause1) has been reported.
Generally, uterine leiomyomas are known to be estrogen sensitive. In fact, both estrogen and progesterone receptors were identified in the lung tissues of our case, which is similar to other cases, suggesting that the pulmonary lesions represented metastatic nodules from benign tumors. The presence of hormone receptor in lung tissues can lead to treatment based on hormonal manipulation with either surgical or medical oophorectomy, including the administration of progesterone2, 12). Anti-estrogen therapy has been found to be effective in individual cases13), or it has been useful for the maintenance of stable disease in another case14). It has also been postulated that BML may be the results of vascular invasion of a uterine leiomyoma or it may be related to intravenous leiomyomatosis15). On the other hand, Kayser et al.7) regarded BML as a slow-growing variant of uterine leiomyosarcoma, but it has a more favorable prognosis.
In a recent cytogenetic study, a monoclonal origin was suggested for both the uterine and pulmonary tumors, and the findings of identical X-chromosome inactivation and a balanced karyotype were consistent with a clonal metastasizing process16). However, in most of the cases, the chromosomal abnormalities associated with BML have been difficult to characterize. In contrast to BML, approximately 25% of the uterine leiomyomas may have a balanced translocation, trisomy 12 and rearrangement of 6p17).
Because of the limited number of therapeutic options and the mismatched results of several reports concerning previously known hormonal therapy, some of the new drugs like Gleevec® (Novartis pharmaceuticals, Basel, Switzerland) have been suggested to have some therapeutic effect on this tumor18). The rationale for the use of Gleevec is based on the recent interest in the c-kit expression in low grade leiomyosarcoma and gastrointestinal stromal tumors and the ability of Gleevec to suppress the expression of c-kit, abl and platelet-derived growth factor. We should focus on the results of the c-kit test because of the histologic similarities between low grade leiomyosarcomas and BML. In our case, the test result for c-kit was negative.
In conclusion, we report here on an interesting case of BML in a 51-year-old woman who had the longest reported period of clinical progression and she was the oldest age Korean patient. This patient was successfully treated with curative excision.