Neutrophil Elastase Causes MUC5AC Mucin Synthesis Via EGF Receptor, ERK and NF-kB Pathways in A549 Cells
Article information
Abstract
Background
Neutrophil elastase (NE) was found to increase the respiratory mucin gene, MUC5AC, although the molecular mechanisms of this process remain unknown. We attempted to determine the signal transduction pathway through which NE induces MUC5AC gene expression in bronchial epithelial cells.
Methods
A fragment of 1.3 Kb MUC5AC promoter which had been cloned into the pGL3-Basic luciferase vector was transfected to the A549 cells. By measuring the luciferase activity, we were able to evaluate the MUC5AC promoter activity in A549 cells. The involvement of mitogen-activated protein kinases (MAPK) was confirmed by Western blotting. To confirm the involvement of nuclear factorkappaB (NF-kB), we used site-directed mutagenesis and electrophoretic mobility shift assay (EMSA) autoradiogram. The MUC5AC mRNA expression was confirmed by RT-PCR.
Results
NE increased the transcriptional activity of the MUC5AC promoter in A549 cells. The increased transcriptional activity of the MUC5AC promoter by NE was found to be associated with increased NF-kB activity. Site-directed mutagenesis showed that the transfection of the mutated NF-kB binding sites from the PGL3-MUC5AC-3752 promoter luciferase reporter plasmid decreased the luciferase activity after NE stimulation. Among the MAPKs, only extracellular signal-regulated kinases (ERK) were involved in this NE-induced MUC5AC mucin expression. RT-PCR also showed that NE increased MUC5AC mRNA. An EMSA autoradiogram revealed that NE induced NF-kB:DNA binding.
Conclusions
These results indicate that human NE induces MUC5AC mucin through the epidermal growth factor receptor (EGF-R), ERK, and NF-kB pathways in A549 cells.
INTRODUCTION
Goblet cell hyperplasia is a prominent feature in many chronic obstructive airway diseases associated with mucus hypersecretion, including chronic bronchitis, bronchiectasis, cystic fibrosis, and bronchial asthma. In these diseases of the airway, neutrophils are recruited into the airways. Hypersecretion from an increased number of goblet cells has been considered to contribute to mucus plugging and airway obstruction1). In the secretions of the airway, MUC5AC is a major mucin which is secreted from the goblet cells of the airway surface epithelium2). Mucins are large molecular weight glycoproteins which are composed of 10 to 20% protein and 80 to 90% complex carbohydrates. Neutrophils release elastase, a serine protease that is found in high concentrations in the airway surface fluids of patients with chronic obstructive airway diseases. Purified neutrophil elastase (NE) was previously shown to be a potent secretagogue for goblet cells in vitro3). In one report, NE increased MUC5AC mucin gene expression and glycoprotein production in respiratory epithelial cells; this increase in MUC5AC gene expression is due to the stability of MUC5AC mRNA stability4). In contrast, the induction of the MUC5AC gene by NE is also related to the reactive oxygen species5). Mitogen-activated protein kinase (MAPK) involves NE-induced morphological changes in human bronchial epithelial cells6). Mammals express at least four groups of MAPK: extracellular signal-related kinases (ERK)-1 and -2, p38 kinases, ERK-5, and c-Jun amino-terminal kinases (JNK 1, 2 and 3), which are also known as stress-activated protein kinases, p38 kinases and ERK-57). MAPKs are a family of proline-targeted, serine-threonine kinases that transduce environmental stimuli to the nucleus8). In this report, we sought to determine the signaling pathway of the human neutrophil elastase (HNE)-induced MUC5AC gene expression in the airway epithelial cells of the airway. We demonstrated that ERK is involved in elastase-induced MUC5AC mucin expression, and also found that epidermal growth factor receptor (EGF-R) and nuclear factorkappaB (NF-kB) are also involved in this signal transduction pathway.
MATERIALS AND METHODS
Cell culture and stimulation
A549 cells, a human pulmonary adenocarcinoma cell line, were grown on 100 mm dishes (Nunc, Denmark) in RPMI 1640 medium containing 10% fetal bovine serum (FBS), penicillin (100 U/mL), streptomycin (100 µg/mL), and HEPES (25 mM) at 37℃ in a humidified, 5% CO2, water-jacketed incubator. At confluence, the A549 cells were maintained for 48 hours in 6-well culture plates at 2×105 cells/well. The cells were then starved of serum for an additional 24 hours and stimulated with HNE (Elastin Products Company Inc., USA) for 30 minutes or with RPMI medium alone (control).
Transient transfections
Methods by which measurement of MUC5AC promoter activity with luciferase as a reporter can be achieved have been reported previously9). A 1.3 Kb-sized fragment of MUC5AC promoter which was cloned into the pGL3-Basic luciferase vector was generously provided by Carol Basbaum (University of California, San Francisco). Prior to transfection, A549 cells were seeded in 6-well tissue-culture plates (2×105 cells/well) and incubated for 48 hours in serum-free medium. The pGL3-MUC5AC-3752pro luciferase reporter plasmid and control pGL3 basic vector were adjusted to 200 ng/µL, and beta-galactosidase was adjusted to 100 ng/µL. The tube designated 'A' contained 300 µL of serum media, 5 µL of pGL3-MUC5AC-3752pro luciferase reporter plasmid, 5 µL of Plus reagent (GIBCO-BRL), and 3 µL of beta galactosidase, while the ube designated 'B' contained 300 µL of serum-free media and 4 µL of LIPOFECTAMINE™ reagent (GIBCO-BRL). Each tube was mixed well at room temperature, and 200 µL of the mixture was added to the wells containing A549 cells. After 5 hours, 1 mL of 20% FBS was added to the wells, and the plates were further incubated for 24 hours.
Luciferase assay
Transfected A549 cells were stimulated with NE for 30 minutes. To examine the effects of the EGF-R tyrosine inhibitor on the transcriptional activity of the MUC5AC promoter, we added 50 µM of AG1478 to the medium 30 minutes prior to stimulation with neutrophil elastase. To examine the effects of MAPK, we added the MAPK/ERK kinase (MEK)-inhibitor, PD98059 (50 µM), and to examine the effect of NF-kB, we added the NF-kB inhibitor, caffeic acid phenethyl ester (CAPE) (20 µg/mL), to the medium 30 minutes prior to stimulation with NE. MUC5AC promoter activity was determined by measuring luciferase activity after the lysing of the transfected cells and normalization by co-transfection with the β-galactosidase expression plasmid, pβgal-control vector (Clontech). β-galactosidase activity was measured in the luminometer (Turner Designs) in accordance with the instructions of the manufacturer of the pβgal-control vector (Clontech). All transfections were performed in triplicate wells; results were reported as emitted light per well (mean±S.D).
RT-PCR
RNA was isolated from cell cultures as previously described10), using an RNA isolation kit (TRIZOL reagent, Invitrogen). The RNA was then reverse transcribed into cDNA. Oligonucleotide primers were designed on the basis of published sequences for human MUC5AC11). The primers for amplifying MUC5AC cDNA were 5'-TCCGGCCTCATCTTCTCC-3' (5'-primer) and 5'-ACTTGGGCACTGGTGCTG-3' (3'-primer). The primers for amplifying glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were 5'-ACCACAGTCCATGCCATCAC-3' (5'-primer) and 5'-TCCACCACCCTGTTGCTGTA-3' (3'-primer). PCR was performed using the standard technique12). The amplification efficiency for MUC5AC was compared with GAPDH, and their ratio was calculated. Statistical comparisons were made using Student's t test.
Immunocytochemistry
Cells grown for 48 hours on 6-chamber slides were stimulated with NE (50 nM) and fixed with 100% methanol for 5 minutes. Cells were incubated with mouse monoclonal antibody (Ab) to MUC5AC (clone 45M1, 1:1,000, New Markers, Fremont, CA) for one hour and were then washed 4 times with phosphate buffered saline (PBS) to remove excess primary antibody. After one hour, plates were washed three times with PBS. The plates were then incubated with biotinylated horse anti-mouse IgG at a 1:2,000 dilution for 30 minutes. Bound antibodies were visualized according to standard protocols for the avidin-biotin-alkaline phosphatase complex method.
Site-directed mutagenesis
The MUC5AC gene fragments for the transcription factor NF-kB binding sites were mutated in order to determine which part is necessary for the initiation process of MUC5AC gene transcription. Two known portions of NF-kB binding sites (base pairs - 973 to - 939 and - 237 to - 203) were present in the MUC5AC promoter. Human MUC5AC regulatory regions were mutated (M1; - 971 to - 939, M2; - 973 to - 941, M3; - 235 to - 203, M4; - 237 to - 205) using a mutagenesis kit (Quick-Change™ Site-directed Mutagenesis Kit; Stratagene) (Table 1). PCR was performed by incubating 1 µL of pGL3 MUC5AC-3752 promoter luciferase reporter plasmid (10 ng/L) with 130 pmole of upstream and downstream primer, 400 µM of total dNTP, 3 µL of Quick solution, and 2.5 units of DNA polymerase in a final volume of 50 µL. PCR was carried out for 10 cycles (30 seconds at 95℃, 60 seconds at 55℃, 60 seconds at 68℃) and finished with an extension at 72℃ for 10 minutes. After PCR, 1 µL of restriction enzyme, Dpn I (10 U/µL), was added to each sample, which was then incubated at 37℃ for one hour. With each mutant, cell transformation to the E.coli was performed with an XL10-Gold E. coli kit (Stratagene). After sequencing, mutated DNA was harvested with Quiagen Midi-prep.
Western blot
A549 cells grown in 6-well culture plates were starved of serum for 24 hours, and were then stimulated with HNE for 30 minutes. In inhibition studies, a selective EGF-R tyrosine kinase inhibitor (AG1478; 50 µM), an MEK inhibitor (PD98059; 50 µM), and an NF-kB inhibitor (CAPE; 20 µg/mL) were added to the medium 30 minutes prior to stimulation with HNE. Cells were then lysed on ice in PBS lysis buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1mM EDTA, 0.02% sodium azide, 1 µg/mL aprotinin, 1 g/mL pepstatin A, 0.2 mM phenylmethylsuflonyl fluoride (PMSF), and 0.6 mM sodium vanadate. The lysate proteins were mixed with the same amount of loading dye, and were then denatured with heating at 95℃ for 10 minutes. The sample proteins (20 µL) were separated by SDS-PAGE in 10% acrylamide gel. The resulting gel was equilibrated in the transfer buffer (48 mM Tris-base, 39 mM glycine, 20% methanol, 0.037% SDS, pH 8.3). The proteins were then electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories), after which the membranes were blocked for one hour with 3% skim milk containing 20 mM Tris-HCl, 150 mM NaCl, and 0.05% Tween 20, at pH 7.5 at room temperature. The membranes were then incubated overnight with primary antibody (1:1,000) in 2% skim milk/Tris-buffered saline with Tween (TBST) buffer. The membranes were washed three times with TBST solution and incubated with horseradish peroxidase-conjugated rabbit antibody (1:2,000) for one and a half hours. After two additional washes, the signal was developed with ECL™ reagent (Amersham-Pharmacia Biotech), and was detected in the X-ray film.
Electrophoretic mobility shift assay (EMSA)
Transfected A549 cells were stimulated with HNE (50 nM) for 30 minutes. The cells were washed with ice-cold PBS and centrifuged at 3,000 rpm for five minutes. Buffer A (0.9 M HEPES [pH 7.9], 1 M MgCl2, 2M KCl, 100 mM DTT, 100 mM PMSF) was added to the cell pellets; the cell pellets were kept on ice for 10 minutes. The cell pellets were again purified after centrifugation at 3,500 rpm and 12,000 rpm, each for five minutes. Buffer C (0.5 M HEPES [pH 7.9], 1 M MgCl2, 2.5 M NaCl, 100 mM EDTA, 100 mM DTT, 4% glycerol) was added to the cell pellets; the cell pellets were allowed to react on ice for 20 minutes. Nuclear extracts were finally isolated after centrifugation at 12,000 rpm for 10 minutes. The primers for EMSA were constructed (sense: 5'-TGTGCTGGGACTGCTCGGACCA-3'; antisense: ACACGACCCTGACGAGCCTGGT) and heated at 95℃ for 10 minutes after mixing the upstream and downstream primers at 1 nmole. The oligonucleotide probes were end-labeled with γ-p32dATP (3,000 Ci/mmol at 10 mCi/mL; Amersham Co., Arlington Heights, IL) in the presence of T4 polynucleotide kinase and were isolated after being passed through a Sephadex G-25 column. Binding reactions containing equal amounts of nuclear protein extract (10 µg) and 35 fmol (~ 50,000 cpm, Cherenkov counting) of radio-labeled oligonucleotide probes were performed for 30 minutes in binding buffer (4% glycerol, 1 mM MgCl2, 0.5 mM EDTA [pH 8.0], 0.5 mM DTT, 50 mM NaCl, 10 mM Tris [pH 7.6], and poly[dI-dC]). For antibody interaction, antibodies specific for NF-kB p65, NF-kB p50, and Rel-C were added to the reaction during 30 minutes of preincubation on ice. Samples were applied to polyacrylamide gels under native conditions in 0.25X TBE buffer (10 mM Tris-HCl [pH 8.0] and 1mM EDTA). The dried gel was exposed to X-ray film with double intensifying screens at -70℃ for 2 to 12 hours. Competitions were performed with 100-fold excesses of unlabeled oligonucleotide in the reaction mixture prior to the addition of the radio-labeled probe. For supershift analyses, 1 µL of each of the antibodies of interest (anti-NFkB p50, anti-NFkB p65, and anti-NF-kB Rel-C) was added to the proteins and left for one hour on ice prior to the addition of the radio-labeled probe.
RESULTS
Neutrophil elastase increases the transcriptional activity of MUC5AC promoter
Before MUC5AC promoter transfection, transcriptional activity was nearly undetectable in a luminometer assay. After transfection, luciferase activity was found to have increased, and 50 nM of neutrophil elastase markedly increased the transcriptional activity in transfectant A549 cells (Figure 1).

Neutrophil elastase (NE) increases the transcriptional activity of MUC5AC promoter. MUC5AC reporter plasmid was transfected to the A549 cells, which then underwent treatment with vehicle or NE for 30 minutes at concentrations of 10, 25, 50, 100, and 200 nM concentrations. The cells were then harvested for measurement of luciferase activities. Significant difference in the transcriptional activity of MUC5AC promoter between control and 50 nM of NE stimulation to transfected A549 cells was detected are found. Values represent the average of six replicates normalized to co-transfected β-galactosidase luciferase activity, with standard deviation indicated with error bars (*, p<0.05 compared with transfection with MUC5AC promoter without NE treatment).
AG1478, PD98059, and CAPE all inhibited the transcriptional activity of MUC5AC promoter
To determine whether the EGF-R-MAP kinase-NF-kB pathway is involved in the NE-induced transcriptional activity of MUC5AC promoter, we added AG1478, a selective tyrosine kinase inhibitor; PD98059, a selective MEK inhibitor; and CAPE, an NF-kB inhibitor for 30 minutes prior to treatment with NE. These inhibitors completely suppressed the neutrophil elastase-induced transcriptional activity of the MUC5AC promoter (Figure 2).

Effects of inhibitors on the MUC5AC promoter activity in A549 cells. The nuclear factorkB (NF-kB) inhibitor (CAPE; 20 µg/mL), MAPK/ERK kinase (MEK) inhibitor (PD98059; 50 µM), and EGF-R inhibitor (AG1478; 50 µM), were added before treatments with neutrophil elastase (NE) (50 nM); all of these inhibitors markedly inhibited the promoter activity of MUC5AC in the A549 cells. Values represent the average of four assays, and standard deviation is provided by error bars (*, p<0.01 compared with transfection with MUC5AC promoter without NE treatment).
Immunocytochemical staining
To determine whether NE increases the secretion of MUC5AC mucin from A549 cells, we performed immunocytochemical staining using the mouse monoclonal Ab to MUC5AC (clone 45M1, 1:1,000 New Markers, Fremont, CA). NE (50 nM) increased the secretion of MUC5AC mucin from A549 cells (data not shown).
Site-directed mutagenesis
Three base pairs of human MUC5AC regulatory regions which were known to be NF-kB binding sites were mutated (base pairs - 971 to - 939, mutant 1; - 973 to - 941, mutant 2; - 235 to - 203, mutant 3; - 237 to - 205, mutant 4) and transfected to the A549 cells. Except in mutant 3, NE (50 nM) did not increase the luciferase activity of the mutated promoter transfectants (Figure 3).

Characterization of the nuclear factorkB (NF-kB) site and NF-kB in MUC5AC mucin induction by neutrophil elastase (NE). Two portions of the NF-kB binding sites of MUC5AC reporter plasmid were mutated (mutants 1, 2 and mutants 3, 4) and transfected into the A549 cells. Three base pairs of human MUC5AC regulatory regions (base pairs -971 to -939, mutant 1; -973 to -941, mutant 2; -235 to -203, mutant 3; -237 to -205; mutant 4) were mutated, as in table 1. Luciferase activity was measured from the control A549 cells and from each mutant-transfected A549 cell during the resting state and after stimulation with NE. Results shown are the average of four replicates, with standard deviation indicated by error bars (*, p<0.05 with respect to controls).
RT-PCR
To confirm the expression of MUC5AC mRNA, RNA analysis was carried out on the A549 cells using RT-PCR. NE (50 nM) increased the expression of MUC5AC mRNA (Figure 4). Signal transduction inhibitors, such as AG1478, PD98059, and CAPE, all suppressed the expression of MUC5AC mRNA by NE.

RT-PCR analysis of MUC5AC expression in A549 cells. Neutrophil elastase (NE) (50 nM) increased the MUC5AC mRNA levels. Pretreatment with nuclear factor-kB (NF-kB) inhibitor (CAPE; 20 µg/mL), MAPK/ERK kinase (MEK) inhibitor (PD98059; 50 µM), or epidermal growth factor receptor (EGF-R) phosphorylation inhibitor (AG1478; 50 µM) suppressed the MUC5AC expression back to the levels of the control. MUC5AC mRNA levels were normalized to glyceraldehydes-3-phosphate dehydrogenase (GAPDH) levels by densitometry. Data is representative of four separate experiments (*, p<0.05 with respect to controls).
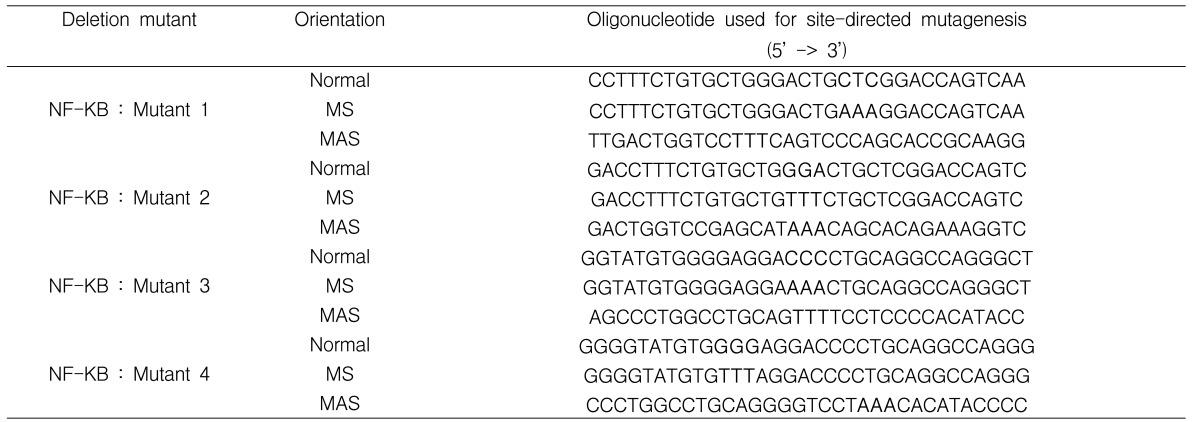
ERK1/2 were required for the activation of MUC5AC promoter by neutrophil elastase
To assess whether MAPK is involved in the NE-induced secretion of MUC5AC mucin in A549 cells, we performed immunoblotting with anti-phospho ERK1/2, anti-phospho P38, and anti-phospho JNK antibodies after stimulating the A549 cells with NE (50 and 100 nM). Only p-ERK1/2 developed; neither p38 nor JNK developed after stimulation with NE (Figure 5). The expressions of p-ERK1/2 after NE stimulation were completely suppressed after treatment with AG1478, PD98059, and CAPE.
Effects of neutrophil elastase (NE) and signal transduction inhibitors on the phosphorylation of extracellular signal-regulated kinases (ERKs), p38 MAPK, and JNKs were determined by Western blotting. NE (50 nM) phosphorylated the ERK1/2, whereas CAPE (20 µg/mL), PD98059 (50 µM), and AG1478 (50 µM) completely inhibited ERK1/2 phosphorylation. NE did not phosphorylate the p38 MAPK or the JNKs.
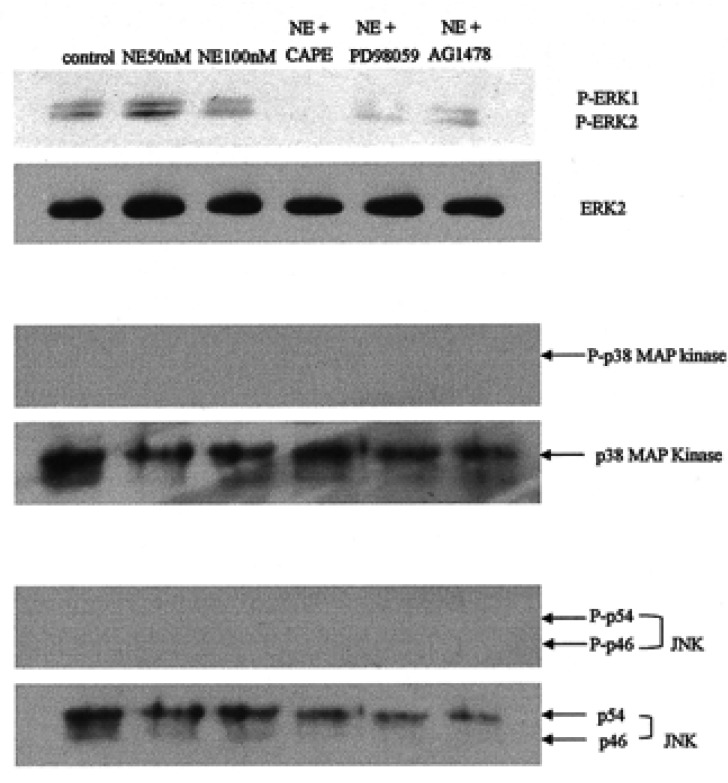
NF-kB is involved in the induction of MUC5AC promoter activity by neutrophil elastase
Next, we assessed the effects of neutrophil elastase on the activation of NF-kB in transfected A549 cells. EMSA showed increased nuclear NF-kB binding with the kB oligonucleotide probe by NE (Figure 6). This binding specificity was confirmed by competition and supershift assays.
Representative electrophoretic mobility shift assay (EMSA) autoradiogram showing the neutrophil elastase (NE) (50 nM) induced nuclear factor-kB (NF-kB): DNA binding. Double-stranded oligonucleotide probes corresponding to the human MUC5AC NF-kB site were incubated with nuclear proteins (10 µg) from A549 cells pretreated with vehicle or NE for 30 minutes. Antibodies (Ab) used for supershift are indicated, as are the oligonucleotides used as cold competitors. NF-kB is involved in the induction of MUC5AC promoter activity by NE. Specificity of binding was ascertained by a supershift of the bands with antibodies to p50, p65, and c-Rel, as well as the reduced intensity of the signals with an excess amount of cold DNA probes (×100).
DISCUSSION
In this study, we found that HNE increased the transcriptional activity of MUC5AC promoter and MUC5AC mRNA expression through the EGF-R ERK NF-kB pathway in A549 cells. Because the concentrations of HNE we used are within the range of airway concentrations of bronchial asthma patients13), the increased mucus secretion of patients with asthma or COPD might result from the presence of NE in the airways. Neutrophils contain elastase at high concentrations (5 mM) in azurophil granules, and possess many biological properties, including pathogen killing, elastin fiber degradation, and MUC5AC mucin induction14). Elastase has been reported to be a more potent secretagogue than other neutrophil proteases and secretory agonists (e.g., cathepsin G and histamine)15). However, until now, the mechanism which links mucin overproduction to HNE has not been well-defined. The A549 cell used in this study is the lung carcinoma cell line, which expressed high levels of MUC5AC mRNA16) and also possesses some of the characteristics of human bronchial epithelial cells17). We hypothesized that HNE-induced mucin production is mediated through the EGF-R-MAP kinase pathway. To confirm this hypothesis, we transfected the A549 cells with the MUC5AC reporter plasmid (from C.Basbaum) and measured the luciferase activity after stimulation with HNE for 30 minutes. The most potent concentration for increasing the MUC5AC promoter activity was 50 nM of HNE. This increased transcriptional activity was markedly inhibited by pretreatment with EGF-R tyrosine kinase inhibitor (tyrphostin AG1478), MEK inhibitor (PD98059), or NF-kB inhibitor (CAPE). The MEK-MAPK transduction pathway was known to be downstream of EGF-R activation, and a selective inhibitor of MEK (PD98059) was reported to inhibit the production of MUC5AC in NCI-H292 cells18). EGF-R is a member of the receptor tyrosine kinase superfamily and is involved in the regulation of proliferation and differentiation, primarily of epithelial cell types19, 20). The stimulation of EGF-R by its ligands, EGF, and transforming growth factor-α (TGF-α) causes MUC5AC mucin expression in airway epithelial cells21, 22). The importance of EGF-R in the production of MUC5AC mucin was demonstrated by the finding that HNE induced MUC5AC mucin production via the proteolytic activation of an EGF-R signaling cascade involving TGF-α in NCI-H292 cells23). In the airways of rats, agarose plugs induce profound goblet cell metaplasia by causing the expression and activation of EGF-R24). Activation of EGF-R is followed by the stimulation of various signaling pathways, including MAPKs p38, c-jun N-terminal kinase (JNK), ERK1/2, and big MAPK (BMK, ERK5), which subsequently results in the activation of various transcription factors (e.g., activator protein-1 and nuclear factor-kB), as well as gene transcription. Among the MAPKs, the three interconnected, relatively well-described pathways are the JNK, ERK, and p38 pathways. Each cascade is composed of at least three enzymes which are activated in series. The ERK1/2 (P44 and p42) pathways are the best characterized of the group. Selective p38 activation, however, was found to be important in the development of human non-small cell lung cancers25). Because the MEK-MAPK transduction pathway is known to be downstream of EGF-R activation and because a selective inhibitor of MEK, PD98059, was reported to inhibit the MUC2 gene expression induced by Pseudomonas aeruginosa26), we attempted to uncover the downstream pathway after EGF-R activation by NE. In our experiments, phosphorylated ERK1/2 were detected after NE treatment, but neither phosphorylated p38 nor phosphorylated JNK were detected by Western blotting. In inhibitor assays, AG1478, PD98059, and CAPE inhibited the development of phosphorylated ERK1/2. The mechanism of inhibition in the development of phosphorylated ERK1/2 by the NF-kB inhibitor, CAPE, is unknown, but we theorized that it might be due to the negative feedback mechanism. Here, we also showed that immunocytochemical staining of the A549 cells demonstrates the presence of MUC5AC mucin in the cytoplasm after HNE stimulation (data not shown). Inflammatory mediators from neutrophils, such as neutrophil elastase, may be present in chronically-diseased airways for prolonged periods of time, and it is believed that this chronic inflammation contributes to the profound airway remodeling observed in patients with long-term respiratory illnesses. For example, long-term exposure of normal human bronchial epithelial (NHBE) cells to IL-13 increases the percentage of Alcian blue/PAS-positive, mucus-producing cells in these cultures. This phenomenon was mediated by TGF-α, a potent ligand for EGF-R, which was rapidly released from NHBE cells in response to IL-1327).
RT-PCR analysis also showed that 50 nM of HNE increased the expression of MUC5AC mRNA; it also showed that this increase was inhibited by pretreatment with CAPE, AG1478, or PD98059. Because CAPE is an inhibitor of NF-kB translocation, NF-kB is likely to be important to the expression of MUC5AC mRNA which is caused by HNE stimulation in A549 cells. NF-kB is a transcription factor which is critical for maximal expression of many cytokines that are involved in the pathogenesis of inflammatory diseases, such as adult respiratory distress syndrome (ARDS) and sepsis syndrome28). NF-kB is activated by a variety of cytokines and mitogens. It is a key regulator of many of the genes which are involved in immune and inflammatory responses29, 30). Activation of NF-kB via Src-dependent Ras-MAPK-pp90rsk is important for Pseudomonas-induced mucin production in epithelial cells31). The NF-kB family of transcription factors consists of homo- or hetero-dimeric subunits of the Rel family, including p65, p50, p52, c-Rel, and Rel-B29). The regions between -973 to -939 and -237 to -203 of the MUC5AC promoter are known to be responsible for the transcriptional activation induced by NF-kB. The promoter regions including the specific NF-kB binding site in the human MUC5AC gene were cloned, and these transcriptional sites were upregulated by bacterial exoproducts32). When these NF-kB binding sites were mutated and then transfected to the A549 cells in this experiment, the transcriptional activity was markedly inhibited in spite of HNE stimulation. These results show that the regions of the MUC5AC promoter between -973 to -939 and -237 to -203 are necessary for both basal promoter activity and NF-kB-induced transcriptional activation. Furthermore, EMSA using an oligonucleotide containing the previously identified MUC5AC NF-kB site demonstrated that nuclear extracts from HNE- treated A549 cells gave more intense bands than did nuclear extracts from untreated cells. For supershift assays, antibodies specific for p50, p65, or the c-Rel subunits of NF-kB were preincubated with nuclear extracts for 30 minutes on ice. The addition of p50, p65, and c-Rel antibody caused a decreased band intensity, which indicated a possible interaction. Thus, it appears that p50, p65, and c-Rel are all involved in the upregulation of MUC5AC by HNE. The specificity of protein binding to radio-labeled oligonucleotide was demonstrated by the addition of a 100-fold excess of unlabeled oligonucleotide. These results demonstrated that the NF-kB transcription factor activates MUC5AC expression through the NF-kB binding sites (-973 to -939 and -237 to -203) in the MUC5AC gene by binding as the p50/p65/c-Rel heterodimer.
It has been found that mucus hypersecretion can be triggered by a variety of factors, including proinflammatory cytokines (e.g., IL-4, IL-9, IL-13), EGF, TNF-α, ATP, bacterial exoproducts, and NE3, 32-35). Mucus is essential because of its role in protecting the airways. In chronic airway disease, however, mucus hypersecretion is an important factor in morbidity and mortality36). NE and oxidant radical are especially important in the pathogenesis of pulmonary emphysema. NE increases the reactive oxygen radicals in lung cells, which contribute to cell death37). NE is also known to increase the MUC5AC mRNA levels by enhancing mRNA stability3) or by inducing reactive oxygen species4). MAPKs may be directly activated by oxidants such as H2O221). Oxidative stress causes the synthesis of MUC5AC mucin in NCI-H292 cells by transactivation of p44/42MAPK and EGF-R18). EGF-R serves a central role as a primary regulator of epithelial function, transducing extracellular signals from its activating ligands into intracellular signaling cascades through dimerization and transphosphorylation catalyzed by the intrinsic tyrosine kinase38). Depending on the triggering stimuli, the signal transduction pathways which control mucin transcription have distinct pathways25, 39). NE also promotes the detachment of the epithelial cells of the airway and changes the morphology of bronchial epithelial cells through the activation of MAP kinase36).
In this experiment, we defined the role of NE in MUC5AC mucin secretion and determined its signal transduction pathways in A549 cells. The MUC5AC mucin synthesis might be caused by both the direct stimulation of NE and the oxidant radicals which were produced from the A549 cells by NE. We speculate that if the signal is mediated by oxidative stress, such as the activation of ERK1/2 by H2O2, antioxidants such as N-acetylcysteine40) will be effective in reducing MUC5AC mucin production. We also showed that, among the MAPKs, only ERK1/2 are involved in the secretion of MUC5AC mucin by neutrophil elastase; p38 and JNK are not involved in this process. These findings suggest that drugs such as Genistein (4'5'7-trihydroxyisoflavone)41), a known inhibitor of ERK1/2, will be effective in decreasing MUC5AC mucin synthesis. We hope that this study will provide potential therapeutic targets in neutrophil-predominant airway inflammation and mucus obstruction of the airways.
ACKNOWLEDGEMENT
The authors thank Dr. CB Basbaum (University of San Francisco, CA, USA) for the kind provision of pGL3-MUC5AC-3752pro luciferase reporter plasmid.
Notes
This experiment was done in part with financial support from the grants of the Catholic University Medical College and the Korean Association of Internal Medicine.