The Cytokines, Interleukin-1β, Interleukin-6 and Interferon-γ Upregulate the Expression of Intercellular Adhesion Molecule-1 (ICAM-1) in Rat Thyroid Cell Line, FRTL-5
Article information
Abstract
Objectives
Recently, the role of adhesion molecules in the immune system has been recognized. ICAM-1 plays an important role in a variety of inflammatory and immune mediated mechanisms, including recruitment and targeting of lymphocytes. We observed the effects of cytokines on expression of rat homologue of human intercellular adhesion molecule-1 in rat thyroid cell line, FRTL-5.
Methods
We have examined expression of rat intercellular adhesion molecule-1 (ICAM-1, CD54), a homologue of human intercellular adhesion molecule-1, by immunocytochemistry (immunoperoxidase staining) in the continuously growing rat thyroid cell line, FRTL-5.
Results
Low level of ICAM-1 expression was noted at basal condition and this basal expression was not influenced by thyrotropin. Expression in rat homologue of ICAM-1 is increased by interferon-γ, interleukin-1β and interleukin-6 with a dose dependent manner.
Conclusion
These results show that a pure line of rat thyroid cells can express an ICAM-1 homologue and this is directly enhanced by cytokines such as rat interferon-γ, human interleukin-1β and interleukin-6. Expression of this homologue is partially responsible for lymphocyte adhesion to thyroid cells, which is likely to be a major event in T cell recognition of thyroid antigens in autoimmune thyroiditis.
INTRODUCTION
Thyroid follicular cells undergoing autoimmune attack express high level of MHC class II molecule1–3). Besides MHC class II molecule, a second group of receptors and ligands, termed adhesion molecules, are involved in initiating and strengthening T cell-target cell interactions. Intercellular adhesion molecule-1 (ICAM-1, CD54) is a cell surface ligand for lymphocyte function associated antigen-14). It plays an important role in a variety of inflammatory and immune mediated mechanisms, including lymphocyte recruitment and targeting, antigen presentation and recognition and lymphocyte toxicity5,8).
ICAM-1 is expressed on thyroid follicular cells of patients with Hashimoto disease7) and cultured thyroid monolayer cells derived from thyroid surgical specimen8). The release of various cytokines at the site of inflammation results in local augmentation of ICAM-1 expression. Although cytokines, such as interleukin-1β, tumor necrosis factor-α and interferon-γ, are known to enhance the expression of ICAM-1 by thyroid cells in vitro8–10), the effects of interleukin-1β and interleukin-6 on expression of ICAM-1 in FRTL-5 cells has not been fully demonstrated.
Recently, the monoclonal antibody reactive with rat homologues of ICAM-1 was developed11). In this study, we used rat thyroid cell line, FRTL-5 to examine the effect of interleukin-1β and interleukin-6 on the expression of ICAM-1. These cytokines were choosen because of their immunomodulatory role within an inflammed thyroid gland.
MATERIALS AND METHODS
1. Cells
The FRTL-5 cell line (Ambesi-lmpiombato, 198612) was obtained from Dr. Kohn (NIH, Bethesda) and was cultured as previously described. The cells were grown in Coon’s modified F-12 medium, supplemented with 5% calf serum and a mixture of six hormones (6H):bTSH; 1 (mU/ml), insulin (10 μg/ml), hydrocortisone (1nM), human transferrin (5 μg/ml), somatostatin (10ng/ml), and glycyl-L-histidyl-L-lysine acetate (10ng/ml) and calf serum (5%). They were passaged every 7–10 days and provided fresh medium every 2 or 3 days.
2. Reagents
Recombinant human interleukin-1β was obtained from R & D systems (purity > 97% ED50=3–10 pg/ml). Recombinant human interleukin-6 (purity > 96%) was obtained from Korean Genetic Engineering Institute. Monoclonoal antibody for immunocytochemistry 1A29 (anti-rat CD54; directed against ICAM-1 homologue) was used as protein A-purified proteins from ascites11) (Seikakaku Corp., Tokyo, Japan).
3. Immunocytochemical Studies
FRTL-5 cells were cultured in Ham’s F-12 medium containing 5% calf serum/6H mix as above. Approximately 104 cells/well were placed on 16 well flat bottom chamber slide (Nunc, Naperville, IL) and maintained for 7 days before fresh medium, containing various concentrations of recombinant human interleukin-1β and recombinant interleukin-6 diluted in medium with 5 hormones (no TSH). Immunocytochemical staining was carried out following peroxidase method. Briefly, at 72 hr after cytokine addition, cells were fixed in cold acetone for 10 min. Monoclonal antibodies diluted in phosphate buffered saline (pH 7.4) containing 0.1% bovine serum albumin and 0.01% sodium azide were used at concentrations predetermined to give optimal staining. Anti-rat ICAM-1 monoclonal antibody was applied for 60 min at room temperature, followed by brief washing in Tris buffered saline, pH 7.6. As a negative control, normal mouse serum at a 1/5,000 dilution was applied. After washing, cells were incubated with diluted biotin labelled anti-mouse Ig antibody. Finally, after intermittent washing, the cells were incubated with ABC reagents (Vector, Burlingame, CA USA). Bound peroxidase was visulized using diaminobezidine as substrate and Meyer’s acid hematoxylin (Sigma Chemical Co) was used as counterstain.
RESULTS
FRTL-5 cells were allowed to grow to 90% confluency in the 16 well chamber slide. The 6H medium was removed and recombinant cytokines in 5H medium were added to duplicate wells for 3 days. In two separate cultures of FRTL-5 cells, low level of ICAM-1 protein expressed under basal conditions. As shown in Fig. 1 unstimulated FRTL-5 cells revealed low levels of ICAM-1 protein but the expression of ICAM-1 protein was upregulated by the stimulation with 10, 100, 1,000 U/ml of interleukin-1β. Similarly, interleukin-6 induced expression of ICAM-1 protein in a dose dependent manner (Fig. 2). The addition of TSH to cells in TSH free conditions for 10 days had no effect on ICAM-1 expression. Rat interferon-γ also increases expression of ICAM-1 by FRTL-5 cells (Fig. 3).

Immunocytochemical staining for rat intercellular adhesion molecule-1 (ICAM-1) in FRTL-5 cells after 3 days culture in various doses of human interleukin-1β (U/ml) (R & D systems) contained 5H medium. Primary antibody for detection of rat intercellular adhesion molecule-1 (ICAM-1) was moncolonal anti-rat ICAM-1 (Seikagaku Corporation, Tokyo, Lot No. 92Y01). And the color reaction was developed with horseradish peroxidase substrate 3′, 3-diaminobenzidine tetrahydrochloride.

Immunocytochemical staining for rat intercellular adhesion molecule-1 (ICAM-1) in FRTL-5 cells after 3 days culture in various doses of human interleukin-6 (U/ml) contained 5H medium. The color reaction was developed with horseradish peroxidase substrate 3′, 3-diaminobenzidine tetrahydrochloride.
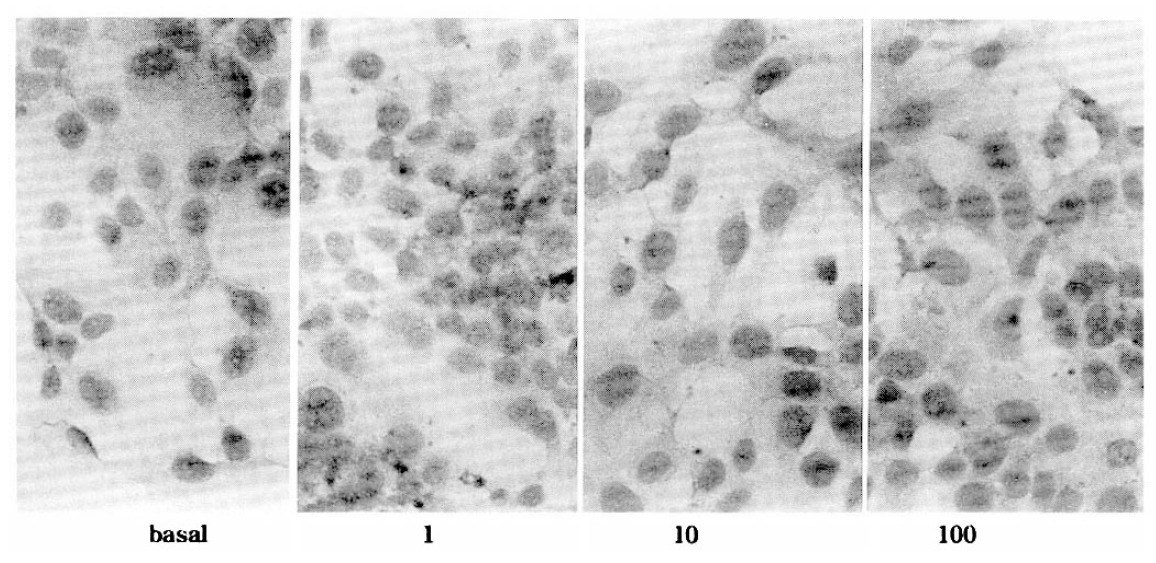
Immunocytochemical staining for rat intercellular adhesion molecule-1 (ICAM-1) in FRTL-5 cells after 3 days culture in various doses of human interferon-γ (U/ml) (Gibco BRL, Lot No. CM1B05, Gaitherberg, MA) contained 5H medium. The color reaction was developed with horseradish peroxidase substrate 3′, 3-diaminobenzidine tetrahydrochloride.
DISCUSSION
The discovery of aberrant expression of MHC class II (DR) molecules on thyroid cells in autoimmune thyroid disease provided new insight in understanding the pathogenesis of organ specific autoimmune disease1–3). Furthermore, it has been suggested that thyrocytes can present an endogenous protein as an antigen and elicit proliferation of specific T cell clones1–3). Although evidence, consistent with the hypothesis of aberrant MHC class II molecule expression, has been observed in many tissues undergoing autoimmune attack, it is uncertain whether this is the cause of the autoimmunity or the resultant T cell activation3). Thus transgenic mice with tissue specific expression of a foreign MHC class II gene showed no spontaneous development of autoimmune activity13,14). Aberrant expression of MHC class II molecules by a variety of cell types may be a response to various cytokines.
Recently, the role of intercellular adhesion molecules in the immune system has been recognized4,5). Thus, cultured human epithelial cells have a capacity to express ICAM-1 antigen in the presence of cytokine and also have a capacity to bind T cells8–10) ICAM-1 antigen on thyroid follicular cells was observed in some surgical specimens of Hashimoto’s thyroiditis. All of these findings suggest that ICAM-1 played an important role in amplification of autoimmune response against thyrocytes. Intercellular adhesion molecule-1 functions as a ligand for the lymphocyte function associated antigen (LFA)-1 and Mac-14,5). The LFA-1/ICAM-1 interaction has been shown to be an important part of lymphocyte adhesion, T cell activation and antigen presentation. The release of certain cytokines at sites of immune responses results in cell activation with local activation of ICAM-1 expression.
A number of cytokines have been detected at both the protein and messenger RNA, including interleukin-1 and interleukin-6, in the chronically inflammed thyroid gland15,16). Regulation of expression of ICAM-1 in the inflammed gland is thus of central importance to understanding the thyroid autoimmune response. The higher basal expression of ICAM-1, seen in the cultured human thyroid epithelial cells derived from thyroid gland affected by Graves’ disease8,9), may be argued to be a nonspecific response to the mononuclear cell infiltrate in this condition, which will secrete cytokines which can induce ICAM-1 expression. Our results with FRTL-5 cells show that the response of thyrocytes to cytokines, interleukin-1β and interleukin-6 is not dependent on pre-existing thyroiditis or on the presence of contaminating mononuclear cells in the primary culture. Interleukin-1 and interleukin-6 production has been demonstrated in monocytes, endothelial cells and rat thyrocytes. So, we think that the basal expression of ICAM-1 in unstimulated FRTL-5 cells is a result of an autocrine effect by FRTL-5 derived cytokines.
Interleukin-1 consists of two separate ligands, interleukin-1β and α. They have a low amino acid identity but bind to the same cell surface receptors to induce a wide range of activities in thyroid cells, such as thyroglobulin synthesis, production of cAMP and iodine organification.
Interleukin-6 is a pleiotropic cytokine that has been shown to be produced by a number of cells, including thyrocytes, and to act on a variety of target cell types. Thyroid epithelial cells from either Graves’ disease and nontoxic goiter were found to produce interleukin-617). The role of interleukin-6 in physiology of thyroid epithelial cells and in expression of certain adhesion molecules has not been fully studied. We demonstrated that the effect of interleukin-6 on expression of ICAM-1 overlaps the effect of interleukin-1 on thyrocytes.
Thyroid epithelial cell expression of adhesion molecule, ICAM-1 in response to inflammatory cytokines, may help explain the pattern of localization of autoimmune response to thyrioid gland. Upregulated ICAM-1 expression by thyrocytes may be of crucial importance in determining the localization, amplification and maintenance of the autoimmune response to thyroid gland.
Acknowledgments
This work was supported by the 1994 Clinical Research Fund of CNUH and the 1993 Alumni Research Fund of Internal Medicine of CNUH. We wish to thank Dr. L. D. Kohn (NIH, Bethesda) for his critical reading of the manuscript.