Absence of a Direct Anabolic Effect of 17 β-Estradiol on Normal Human Bone Marrow Stromal Cells
Article information
Abstract
The effects of 17 β-estradiol on the proliferation and differentiation of cultured normal human bone marrow stromal cells were investigated. Treatment of 17β-estradiol at the concentration of 10−6∼10−10 M for either 48 hours or 7 days did not affect [3H] thymidine incorporation. 17 β-estradiol (10−8 M) treatment for 4 or 7 days also failed to stimulate alkaline phosphatase activity. Similarly, incubation with 17 β-estradiol (10−8 M) for 48 hours did not increase the incorporation of [3H] proline into collagenase digestible protein and noncollagen proteins and secretion of IGF-I and IGF binding proteins in human bone marrow stromal cells.
Present data indicate that 17β-estradiol does not have a direct effect on cultured normal human bone marrow stromal cells. With previous findings that estradiol elicits few effects on normal human osteoblasts, our results strongly suggest that estrogen does not have a direct anabolic effect on normal human osteoblast lineage. Therefore, the in vivo estrogen effects may be entirely through an antiresorptive mechanism or, if any anabolic role of estrogen is present, it must be indirect and mediated by other hormones or local factors.
INTRODUCTION
Estrogen deficiency following menopause, oophorectomy or prolonged amenorrhea is associated with bone loss. The bone loss and associated risk of fractures can be prevented or slowed by estrogen replacement therapy1,2). However, the cellular and molecular mechanisms by which estrogens exert their protective action on bone remains unclear.
In the in vivo rodent model, estrogen was found to inhibit bone resorption as well as to stimulate bone formation3,4). In addition, the demonstration of estrogen receptors in human osteoblasts and osteosarcoma cells has raised the possibility that estrogen may have direct anabolic effects on osteoblasts5–8). However, conflicting results have been reported. In transformed osteoblastic cell lines and in normal animal or human osteoblast-like cells, estrogen treatment has been reported to induce various effects; proliferation is stimulated9–12), suppressed13,14) or not changed15) and differentiation stimulated6,11–13) or not changed14,15). The reason for these divergent and conflicting results is uncertain but probably relates to differences in responses of various model systems.
Since bone marrow stromal cells were shown to be a source for the precursors of osteoblasts16), estradiol may exert the bone formation effect through the preosteoblasts present in bone marrow stroma. Unlike osteoblasts, bone marrow stromal cells are more actively proliferating and differentiating and may have better responses to estradiol. Studies using bone marrow stromal cells, however, are rare. Therefore, we studied the effects of 17 β-estradiol on the proliferation and differentiation of cultured human bone marrow stromal cells.
METERIALS AND METHODS
1. Bone Marrow Stromal Cell Culture
Human bone marrow stromal cells (hBMSC) were isolated from the ribs which were obtained at the time of open thoracotomy. The ribs were excised aseptically, cleaned of soft tissues, opened longitudinally and exposed bone marrow was flushed out several times using culture medium. Flushed bone marrow was centrifuged at 1400 rpm for 10 minutes. Cell pellets were resuspended in culture medium and bone marrow cells were obtained by Ficoll/Hypaque gradient centrifugation. The cells were seeded in 75 cm2 plastic culture flask at a density of 4 × 105 cells/cm2 using α MEM containing 10% fetal bovine serum (FBS, GIBCO) and penicillin and streptomycin (100 u/ml and 100 μg/ml; Sigma). The medium was changed twice a week from the second week and human bone marrow stromal cells were grown to 80% confluence and subcultured using conventional techniques employing 0.01% trypsin and 0.05% EDTA.
For subcultured cells, phenol red-free medium supplemented with 10% (vol/vol) charcoal stripped (CS) serum, penicillin (100 u/ml) and streptomycin (10 μg/ml) were used. All assays were performed on first passaged cells.
2. Proliferation Assay
Proliferation of hBMSC was assessed by measurement of [3H] thymidine (New England Nuclear, Danvers, MA) incorporation. Cells were subcultured in 24-well plates at a seeding density of 2×104 cells/well with standard medium containing 10% FBS and penicillin-streptomycin. The next day, cells were washed with phosphate buffered saline (PBS) and medium was replaced with phenol red-free medium containing 10% CS serum with or without 17 β-estradiol for 48 hours or 7 days. [3H] thymidine was added at 1 μCi/ml for the final 4 hours and the incorporation of [3H] thymidine into trichloracetic acid-precipitable material was measured by standard methods17).
3. Alkaline Phosphatase Activity
hBMSC were seeded into 12-well plates at a density of 4×104/well and treated as above. After 4∼7 days exposure to 17 β-estradiol (10−8 M), the medium was removed and the cell layers were washed and assayed for alkaline phosphatase activity by measuring P-nitrophenyi phosphate (Sigma) hydrolysis18). The data were normalized to represent the alkaline phosphatase activity as nmoles/minute/mg protein.
4. Collagen Synthesis
Collagen synthesis was measured by the incorporation of [3H] proline (Amersham, Arlington Heights, MA) into collagenase (type VII, Sigma) digestible protein. Cells were subcultured into 6-well plates at a density of 6×104/well as above. When cells were confluent, medium was replaced with phenol red-free medium, containing 100 μg/ml ascorbic acid, 100 μg/ml β-aminopropionitrile fumarate and with or without 17β-estradiol (10−8 M) for 48 hours. [3H] proline (5μCi/ml) was added for the last 2 hours. The medium was removed and the cell layers harvested by scraping in 2.5 mM N-ethylmaleimide, 0.2 mM phenylmethylsulfonyl fluoride, 2.5 mM Na2EDTA and 1 mg/ml bovine serum albumin and sonication for 10 sec. Trichloracetic acid was added to a final concentration of 15% and sample were kept at 4°C overnight. The next day, samples were centrifuged at 3000×g for 20 min at 4°C and precipitates were washed 3 times with 5% TCA and dissolved in 50 μl of 1M NaOH. The solubilized pellets were brought to 1 ml in solution containing 0.1 M HEPES, pH 7.3, 3.5 mM CaCl2 and protease inhibitors described above. Samples were divided into equal aliquots and incubated with or without bacteria collagenase and the incorporation of [3H] proline into collagenase digestible protein (CDP) and noncollagen protein (NCP) was determined and % collagen synthesis was calculated19).
5. IGF-I assay
Human bone marrow stromal cells in p-150 culture dishes were grown to confluence. The cells were washed and incubated in phenol red-free medium containing 10% CS FBS for one day followed by serum and phenol red-free medium (30 ml) containing either vehicle or 17 β-estradiol (10−8 M) for 2 more days. Conditioned media (28 ml) were harvested and stored at −20 °C until assay. For IGF-I analysis, the conditioned media were treated with glacial acetic acid (final concentration 4%) and incubated at room temperature for 30 min. The solutions were applied to Sep-Pak C18 columns which had been washed sequentially with 5 ml each of water, methanol, and isopropanol, and 30 ml 4% acetic acid. The columns were washed with 20 ml of 4% acetic acid and IGF-I eluted by 2 ml of 75% ethanol in 0.1 M acetic acid twice. The ethanol/acetic acid eluates were dried in Speed Vac and the residues analyzed for IGF-I by using RIA kit obtained from Nichols Institute.
6. IGF Binding Proteins Analysis
2 ml of the conditioned media described in IGF-I assay were aliquoted (0.5 ml/fraction) and freeze dried. The residues were dissolved in 100 μl sample buffer in the absence of reducing agent. Samples containing the same amount of proteins were applied to SDS-PAGE. Western blots using nitrocellulose membranes were performed and IGF binding proteins (IGF BP) were measured by the ligand binding analysis using 125I-IGF-I20).
7. Statistics
Statistical analyses were performed using Student’s unpaired t-test.
RESULTS
In the present study, 17 β-estradiol treatment at a concentration of 10−6–10−10 M for 48 hours did not affect [3H]thymidine incorporation of cultured hBMSC (Fig. 1). Prolonged treatment (7 days) showed similar results (data not shown). The addition of 10−8 M 17 β-estradiol to hBMSC cultures for 4 days resulted in only mild elevations of alkaline phosphatase activity (630±167 nmoles/mg protein/min for control vs. 750±224 nmoles/mg protein/min for 17 β-estradiol treated cells), which were not significantly different from control value (Fig. 2). No difference was also observed in the alkaline phosphatase activities between control and 17 β-estradiol treated groups after 7 days treatment (Fig. 2). 17 β-estradiol treatment for 48 hours did not increased incorporation of [3H] proline into CDP and NCP (Fig. 3). % collagen synthesis in estradiol treated cells was 12.97 ± 1.72%, which was not significantly different from control cells, 10.70 ± 1.78%. The secretion of IGF-I (Table 1) and IGF BPs (Fig. 4) into the conditioned media was also not affected by 17 β-estradiol treatment at doses of 10−7–10−9 M.

The effect of 17 β-estradiol on [3H] thymidine incorporation of human bone marrow stromal cells. Test agent was added for 48 hours. Each bar represents mean±SD of 8 determinations. No effect of 17 β-estradiol on human bone marrow stromal cells was observed.
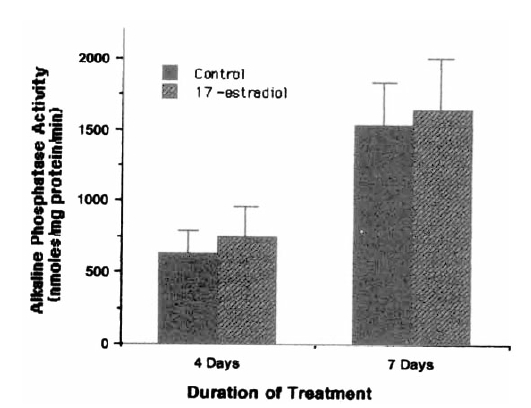
The effect of 17 β-estradiol (10−8 M) on alkaline phosphatase activity of human bone marrow stromal cells. Each bar represents mean±SD of 6 determinations.

The effect of 17 β-estradiol (10−8 M) on total protein (T.P.), collagenase digestible protein (C.D.P.), noncollagen protein (N.C.P.) and % collagen synthesis of human bone marrow stromal cells. Each bar represents mean±SD of 6 determinations.

The effect of 17 β-estradiol (10−8 M) on the secretion of IGF binding proteins in the human bone marrow stromal cells. Cells were treated with vehicle or 17 β-estradiol (10−8 M) for 7 days. The cells were washed and incubated for two additional days in serum free medium containing test reagent. Conditioned media were harvested and freeze dried. Equal amounts of proteins were applied to SDS-PAGE in the absence of reducing agent. IGF binding proteins were analyzed by performing ligand binding after Western blot analysis using 125I-GF-I.
DISCUSSION
Our data showed no observable effects of 17β-estradiol on cell proliferation, alkaline phosphatase activity and collagen synthesis of human bone marrow stromal cells in vitro. These results are consistent with the observations of Keeping et al. who found no major effects of 17 β-estradiol on the proliferation and differentiation of normal human osteoblasts15), but are in contrast to the report of Benz et al. that estradiol increases the steady state α 1 (1)-procollagen mRNA in human osteoblast cell line GB688 which contains high estradiol receptors21) and the report of Scheven et al. that estrogen stimulates both proliferation and differentiation of normal human osteoblasts11).
Since human osteoblasts used in experiments so far were obtained from adult bones, they might have a lower growth rate and be more differentiated than fetal or neonatal bone derived cells. Thus it was suggested that negative results in human osteoblasts could be due to mature osteoblast phenotype. However, human bone marrow stromal cells, less diffenentiated and immature osteoblast precursors, neither responded to estradiol in this experiment, suggesting that the degree of cellular differentiation does not seem to influence estrogen response.
Although estrogen receptors in human osteoblasts and osteosarcoma cells have been demonstrated, the concentration of estrogen receptor in the human osteoblasts is very low in most cases and may not be detectable in many patients22,23). It has been demonstrated that the response to estrogen treatment is dependent on the number of estrogen receptors in a hyperbolic fashion (Webb et al. 1992). Moreover, most of the experiments which showed estrogen effects were performed in transformed cell lines or non-human neonatal osteoblasts which have higher concentration of estogen receptors6,9,10,12–14). It has also been demonstrated that estrogen receptors are present in murine bone marrow stromal cells and cell lines24). Similar to human osteoblasts, it is unclear whether the number of estrogen receptors in human bone marrow stromal cells is low. Whether negative responses in human osteoblasts and bone marrow stromal cells were due to low number of estrogen receptors present in these cells remains to be established.
We also did not observe any effect of estrogen on IFG-I and IGF BPs secretion in human bone marrow stromal cells. The inability to stimulate IGF-I and IGF BPs in these cells by estradiol is consistent with the observation that estradiol has no effect on proliferation of these cells. However, these are in contrast with the reports using fetal or neonatal rat calvarial cells or osteogenic sarcoma cells UMR 1069,25–28). In these rodent-derived osteoblasts, estradiol increased transcription of IGF-I gene and synthesis of IGF-I. Estradiol also regulates IGF BP-2 concentration depending on doses in rat calvarial cells28). The difference between our results on human bone marrow stromal cells and those reported using rodent cells could be due to different species but not to the degree of differentiation of osteoblasts since we also found no effect on IGF-I and its binding proteins in human osteoblasts by estradiol treatment (data not shown). The lack of estrogen effect on IGF-1 and IGF BPs secretion in human bone marrow stromal cells may also derive partly from the low number of estrogen receptors in these cells.
Based on our data and other reports on human cells, we conclude that 17 β-estradiol does not have a direct anabolic effect on normal human osteoblast lineage. To increase bone formation by estrogen, if any anabolic role of estrogen is present in the human, human osteoblast lineage must behave differently from transformed cell line or animal osteoblast lineage. The in vivo anabolic estrogen effects in human may be indirect and mediated by other hormones or local factors.
Notes
This work was supported by a grant from the Asan Institute for Life Science.
