Effect of Angiotensin Converting Enzyme Inhibitor on Collagen Production by Cultured Mesangial Cells
Article information
Abstract
Cultured mesangial cells (MC) express renin mRNA and generate angiotensin I, supporting the action of local renin-angiotensin system. Also angiotensin II may act like a growth factor and was reported to increase collagen production (CP) in cultured MC. Angiotensin converting enzyme inhibitor is suggested to attenuate development and advancement of glomerulosclerosis, mainly with its hemodynamic effects. Therefore, we investigated the direct effects of enalapril (E) on CP by cultured MC. Rat MC were cultured in DMEM media alone, or containing high glucose (HG: 25 mM) or soluble immune complex (IC) prepared with bovine gamma globulin (BGG) and anti-BGG, with or without E (0.2 ug/ml). CP was determined after 24 h by [3H] proline incorporation method. E significantly reduced CP by 43% in medium as compared with control (C)(C: 37,210±4,200 vs C+E: 21,350 ± 5,080 cpm/well, p<0.01). CP in medium increased in the presence of HG (123% of C) or IC (147% of C), which was, however, prevented with E (HG + E: 105% of C, IC+E: 116% of C). There were no differences of CP in cell layer between C (3,490±220 cpm/well) and C+E (3,340±190 cpm/well), and also no changes after, addition of E in HG or IC groups. In conclusion, E directly attenuates CP by MC, even in the presence of HG or IC, independently of its hemodynamic effects.
INTRODUCTION
It’s recently been suggested that the glomerular mesangial expansion may be the common pathway into the development of glomerulosclerosis in several glomerular diseases, such as immunemediated glomerulonephritis and diabetic nephropathy1,2). It’s reported to be due to the synthesis and accumulation of extracellular matrix proteins (ECM) such as collagen3–9).
Since the methods of mesangial cell culture were established10), the mesangial cells (MC) have been observed to proliferate or produce ECM in response to injurious stimuli2,3,10) and also to secrete biologically active substances such as cytokine growth factors as effectors cells11–16). Especially, it’s interesting to determine whether high glucose or immune complex (IC) could exert any effects on MC3,17–20).
MC were reported to express renin-like enzyme activity and generate angiotensin I21,22). Angiotensin II was observed to increase collagen production in cultured MC23) and, therefore, may act as a growth factor. Also, angiotensin converting enzyme (ACE) inhibitor has been suggested to attenuate glomerulosclerosis, probably mainly through its hemodynamic effect24–27), which remain controversial with recent studies28–30).
Therefore, the direct effect of ACE inhibitor, enalapril, on MC were investigated from the aspects of collagen production or DNA synthesis. Also, It was examined whether soluble IC or high glucose exert any effects on cultured MC, and these changes are modulated by enalapril in vitro.
METHODS
Isolation and identification of rat glomerular MC: Glomeruli were isolated from Sprague-Dawley rats using techniques previously described10,14). Collagenase (GIBCO Laboratories, Grand Island, NY, USA)-treated glomeruli were plated on culture dishes in DMEM media containing 17% heat-inactivated fetal bovine serum (FBS), glutamine, penicillin, streptomycin, amphotericin and insulin. Near confluent cells in third to fourth passage were used in these studies. The cells have prominent intracellular myosin fibrils and were negative with antibodies (Becton Dickinson, Mountain View, CA, USA) to common leukocyte antigen and factor VIII by immunofluorescent staining. The cells were capable of growth in D-valine substituted medium and were not sensitive to puromycin.
Experimental groups: the medium was replaced according to the experimental design shown as follows. 1) Control, 2) Enalapril group; enalapril 0.2ug/ml, 3) IC prepared with bovine γ-globulin (BGG) and rabbit IgG anti-BGG at five times excess antigen as previously described17), 4) IC+enalapril group, 5) High glucose; 25 mM glucose, 6) High glucose+enalapril group.
Collagen and non-collagen protein production: De novo collagen synthesis was measured by the incorporation of {3H} proline into collagenase-digestible material as described31). MC were plated at 1×105 cells per well in 6-welll plates in basal medium supplemented with 17% FBS and 5.6 mM (100 mg/dl) glucose. After 72 hr of starvation with serum-free medium, the medium was again changed to medium with 0.2% FBS, 5.6 mM (100 mg/dl) glucose, 50 μg each of sodium ascorbate and β-aminopropionitrile, and the indicated amount of various materials according to the experimental design as mentioned above. The cells were labeled with 5μCi of {3H} proline (Amersham Corp., Arlington Heights, IL, USA). After 24 hr incubation, the proteins in cell and medium were precipitated with 2 ml of 10% TCA and 1% tannic acid. The washed precipitates were dissolved in 0.1 N NaOH and neutralized, and the solubilized proteins were digested with 100 units of highly purified collagenase (GIBCO) in 0.1 M Tris-buffer (pH = 7.6) containing 10 mM CaC12 and 20 mM N-ethyl maleimide for 1 hr at 37°C. Collagenase digestible protein and non-collagenous protein were separated with TCA and tannic acid and the radioactivity of each fraction was determined.
Thymidine incorporation assays: Cells were plated at 1×104 cells per well 96-well plates in DMEM medium containing 17% FBS. One μCi per well of {3H} thymidine (New England Nuclear, Boston, Massachusetts, USA) was added after the medium was replaced according to the experimental design. After 16 hr incubation, the contents of each well were counted in a liquid scintillation counter32).
Statistical Analysis
The results are expressed as mean ± SEM. Statistical evaluation of the data was performed using a Student’s test and ANOVA. Probability values <0.05 were considered significant.
RESULTS
Collagen production in medium by MC in Enalapril group was significantly lower than Control for the first 24 hours and was 57% of control (Enalapril group: 21,350±5,080 vs Control: 37,210±4,200 cpm/well, n = 6, p<0.01) (Table 1, Fig. 1). No differences of non-collagen production in medium were noticed between the two groups. Therefore, the ratio of collagen/total protein production was lower in Enalapril group than in Control. However, there were no differences of collagen production in cell layer between Control (3,490±220) cpm/well) and Enalapril group (3,340±190 cpm/well).

Collagen production in medium by mesangial cells (Mean ± SD) was significantly reduced by enalapril (*p<0.01 vs control).
Collagen production in medium was significantly higher in the presence of IC or High glucose (147% and 123% of Control, respectively) than in Control (p<0.05) (Fig. 2). But, IC or high glucose-induced increases in collagen production were significantly prevented with the addition of enalapril (116% and 105% of Control, respectively, p<0.05).
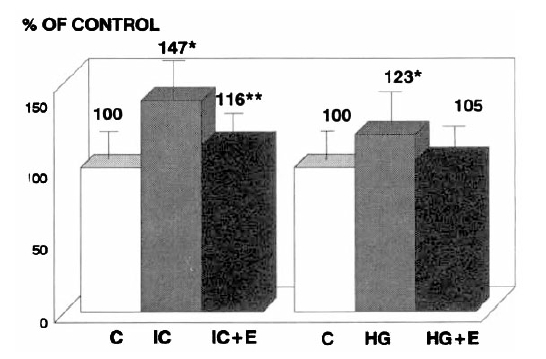
The immune complex or high glucose-induced increases in collagen production in medium were prevented by enalapril (% of mean control value). C: control, IC: immune complex, HG: high glucose, E: enalapril. *p<0.05 IC vs C or IC + E, HG vs C or HG + E **p<0.05 IC + E vs C
When cultured in soluble IC or high glucose, the thymidine incorporation of mesangial cells was decreased as compared with control (63.5% and 72.6% of control, respectively, p<0.05) (Table 3). The addition of enalapril, however, exerted no effects on these changes in IC or High glucose groups.
DISCUSSION
According to these experiments, ACE inhibitor, enalapril, could directly reduce collagen production by MC in vitro. This supports the recent studies that ACE inhibitor could prevent the progression of renal disease in the absence of hemodynamic effects. Also, it suggests that there is the local renin-angiotensin system in cultured MC which enalapril could inhibit.
It has been demonstrated that angiotensin II as a growth factor can induce cellular hypertrophy and also proliferation in MC23,33–35). Angiotensin II directly stimulates the synthesis of extracellular matrix proteins, mainly type I collagen23). Also angiotensin II was observed to induce the synthesis of interleukin-636) and platelet activating factor37) in MC, offering multiple possibilities for the regulation of autocrine and/or paracrine effects of this peptide. Therefore, it’s also possible that the effects of angiotensin II to stimulate collagen production may, in part at least, be mediated by these growth factors. Recently, AT1-angiotensin II receptor was observed to be expressed in MC of human and animals38,39). Current evidences suggest that the AT1 receptor is coupled via G proteins to traditional signal transduction mechanisms such as stimulation of phospholipase C, calcium mobilization and inhibition of adenylate cyclase40).
On the other hand, high glucose induced increases in collagen production by MC in these experiments, as previously reported19,20). High glucose may activate the polyol pathway increasing the glycosylation of proteins by nonenzymatic means, or stimulate second messenger pathways resulting in alterations in the synthesis or degradation of extracellular matrix proteins41). Also, IC stimulated collagen production by MC in these experiments. Soluble IC was previously observed to bind to cultured MC through FC receptor and to activate MC17). While high glucose was related to transforming growth factor-β42), IC were also reported to stimulate the synthesis of interleukin-1 and −618,43). All of these factors could induce the synthesis of extracellular matrix proteins44,45). Therefore, our study complements the reports that collagen synthesis and accumulation leading to mesangial expansion and/or late glomerulosclerosis was increased in IC-mediated glomerulonephritis or diabetic nephropathy models4,5,9,46). According to these experiments, enalapril can reduce the increase in collagen production induced by IC and high glucose. While the underlying mechanisms remain to be delineated, it may be through the inhibition of generation of angiotensin II and related to the above pathways or interaction with growth factors.
When cultured in soluble IC or high glucose in these experiments, the thymidine uptake by MC was decreased, and the addition of enalapril, however, exerted no effects on these changes. The proliferate effects of angiotensin II have not been confirmed by all investigators33).
In conclusion, ACE inhibitor, enalapril, could directly reduce collagen production by MC, also induced by IC or high glucose, irrespective of its hemodynamic effects. Further studies are necessary to investigate the intracellular mechanisms on the levels of collagen gene expression and the interaction of some sutocrine growth factors by using this culture model.
Acknowledgements
This study was supported by the Chong-Ram grant in 1990 from the Korean Association of Internal Medicine


