Pulmonary Embolism as the Initial Manifestation of Large Cell Lung Cancer:
– A Case Report with Review –
Article information
Abstract
Lung cancer is known as a risk factor of pulmonary embolism. We experienced a case of pulmonary embolism combined with pleural effusion and pleuritic chest pain as the initial manifestation of large cell lung cancer, which is a relatively rare cell type of lung cancer in Korea.
We report it with a review of the literature.
INTRODUCTION
Pulmonary embolism is defined as the impaction of material into branches of the pulmonary arterial bed. The annual incidence in the United States has been estimated at approximately 630,000.1) About one third of the episodes are fatal, with nearly all of the fatalities either sudden or undiagnosed during life2–4).
There are several clinical risk factors for pulmonary embolism, of which cancer has been found to be the second most common instance of pulmonary embolism discovered at autopsy. Prevalence of pulmonary embolism is 20% in association with cancers of the lung5). Large cell carcinomas of the lung acount for 16 to 20 percent of bronchogenic carcinomas6). In Korea, these account for 4.5 to 9 percent7,8).
Recently we experienced a case with pulmonary embolism as the initial manifestation of large cell lung cancer in a 38-year-old Korean man. This report also reviews the pathogenesis of pulmonary embolism and the possible relationship with lung cancer.
CASE REPORT
A 38-year-old man was admitted to hospital because of dyspnea and chest pain. He had been relatively well until one month ago, when he suffered from pain on both legs. He was treated with muscle relaxants at a local clinic with mild symptomatic improvement. One day before admission, dyspnea and chest pain abruptly developed. The pain was localized on the right lower anterior and lateral chest and pleuritic. These symptoms became more severe.
The family and past medical history were not contributory.
On admission, blood pressure was 120/80 mmHg, pulse rate 94/min, temperature 37.2 C, and respiration was shallow and rate was 56/min. On physical examination, he was alert but in acute distress. The conjunctiva was pink, and sclera was white. On auscultation of chest, breathing sounds were normal, and the heart sound was regular without murmur. Examination of the abdomen was not remarkable. Further examination revealed no abnormal finding. Laboratory studies included hemoglobin 15.6 gm/dl, hematocrit 44.6%, WBC 21,100/mm3 with 80% neutrophils and 15% lymphocytes, platelet 261,000/mm3, total serum bilirubin 0.6 mg%, ALT 19.1 IU/L, AST 47.4 IU/L and albumin was 4.3 gm%. The concentration of electrolytes was normal and urinalysis was negative for protein and revealed only 20–25/HPF of RBC. At room air, arterial blood gas analysis showed pH 7.368, PaCO2 38.4 mmHg, PaO2 35.0 mmHg, HCO3 22.1 mmol/L. The CEA and alpha feto protein were 6.42 ng/ml and 5 ng/ml, respectively. Chest P-A showed blunting of right costophrenic angle, accentuation of pulmonary vascular markings, and slight enlargement of right superior mediastinum (Fig. 1).
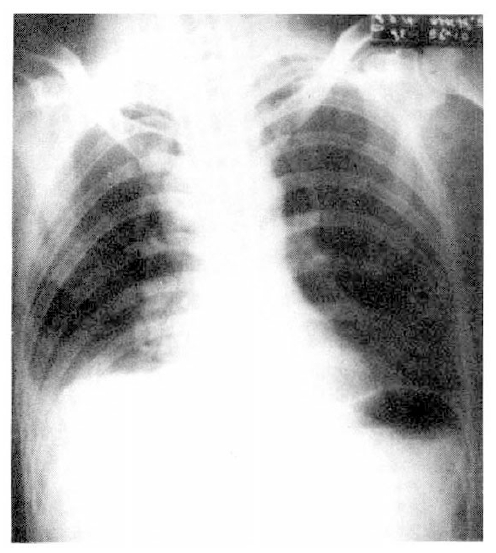
Chest P-A shows blunting of right costophrenic angle, accentuation of pulmonary vascular markings, and slight enlargement of right superior mediastinum
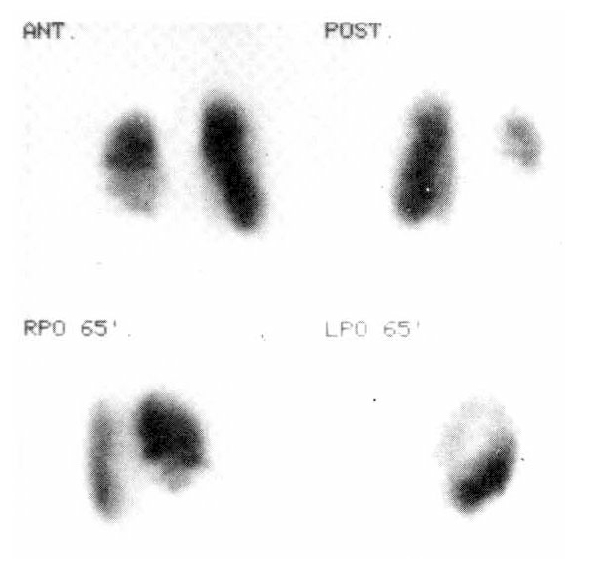
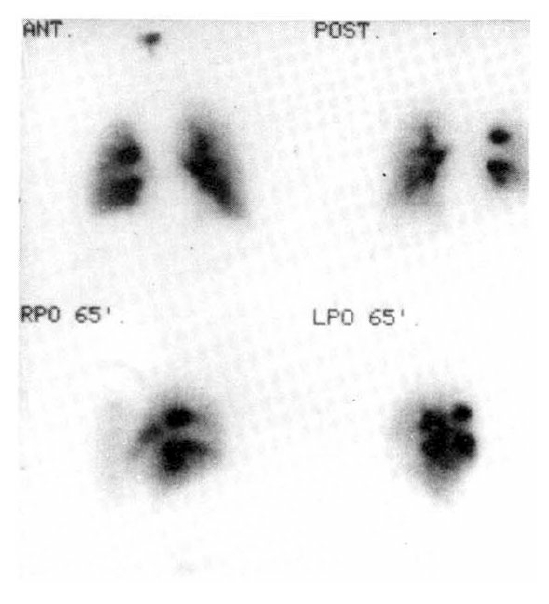
In order to demonstrate pulmonary embolism, a perfusion and ventilation lung scan were taken. The perfusion lung scan showed multiple segmental perfusion defects in right lower lung (Fig. 2), but the ventilation lung scan showed no significant ventilation defect, suggestive of V/Q mismatch on right lower lung (Fig. 3). Impedance plethysmogram showed no abnormal finding on both lower legs. Heparinization started with a usual dose of 5,000 unit bolus intravenously, and 15,000 unit intravenously for 24 hours.
In spite of persistent heparinization, chest pain and dyspnea became more aggravated, and right neck swelling developed 20 days after admission. Perfusion superior vena cavogram showed delayed perfusion in superior vena cava. Neck sonogram revealed soft tissue swelling in neck, but there was no evidence of thrombosis. A computerized tomogram of the chest showed a 2 cm sized low-density mass with irregular margin and minimal pleural effusion in the left lower lung, and enlargement of ipsilateral mediastinal lymph nodes. SVC was partially filled with a small, low-density mass, which was suggestive of a thrombus (Fig. 4).
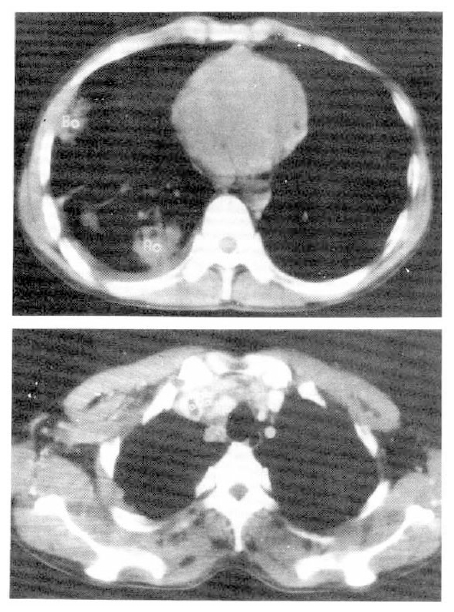
Chest CT scan shows an irregular marginated non-enhancing low-density mass with pleural effusion in the left lower lung, and ipsilateral mediastinal lymph nodes enlargement. And round, small, lower-density was detected in SVC, suggestive of thrombosis of SVC.
At 26th admission day, 1×1 cm sized, non-tender, soft, and round lymph node was palpated on left supraclavicular area. Fine needle aspiration cytology of left supraclavicular lymph node revealed a few large atypical cells with pleomorphic nuclei, prominent nucleoli and abundant cytoplasm. Most of the cells were dispersed individually, but a few clusters were also found. Some of the cells disclosed phagocytoses of the neutrophils (Fig. 5). Histologic finding of the lymph node by surgical biopsy showed sinusoidal invasion of large anaplastic cells. Each tumor cell had large hyperchromatic nucleus and abundant eosinophilic or vacuolated cytoplasm. Their nuclei had one or more prominent nucleoli and occasional bizarre forms. There were frequent mitoses and phagocytic activity of the tumor cells. Many neutrophils were infiltrated among the tumor cells (Fig. 6).
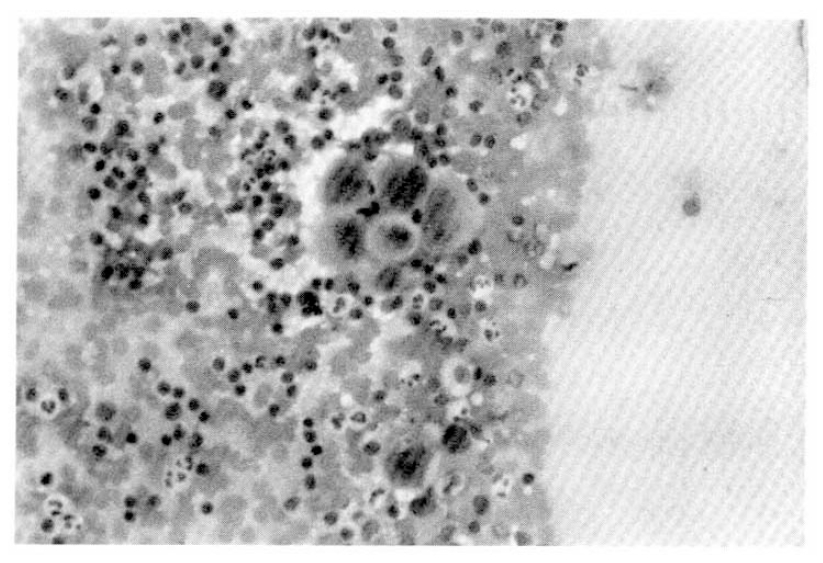
Fine needle aspiration cytology shows a cluster of large atypical cells with prominent nucleoli on inflammatory background.
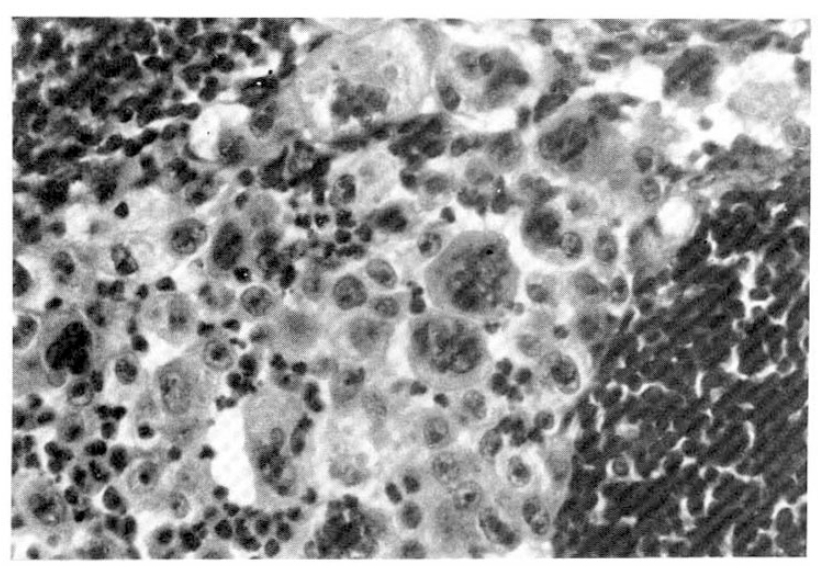
Histologic findings of lymph node show sinusoidal infiltration of large cell carcinoma with many neutrophils
Radiation therapy was applied on mediastinum and right neck with improvement of chest pain, dyspnea, and swelling of right neck. The follow-up perfusion lung scan showed no perfusion defect in right lower lung. Anticoagulation for embolism was changed with warfarin and he was discharged.
He was readmitted because of epigastric discomfort and dyspnea after 2 weeks. The chest P-A showed blunting of both costophrenic angles, suggestive of bilateral pleural effusion. Ultrasonogram also showed bilateral pleural effusion and ascites. In spite of thoracentesis, dyspnea was not improved. EKG showed low voltage and electrical alterance on all leads and echocardiogram revealed severe pericardial effusion (Fig. 7). The patient died on the 4th day after admission due to cardiac tamponade in spite of pericardiocentesis.
DISCUSSION
Because the association between malignancy and pulmonary embolism has been recognized and reemphasized9,10), we must consider malignancy as the risk factor of thrombosis in cases of pulmonary embolism without definite risk factors. Furthermore, thrombosis of upper extremities without any intravenous devices strongly signified the malignancy as risk factor in medical patients11). In this case, at first we did not find any risk factor of pulmonary embolism including trauma and malignancy. Because of his denial of weight loss and symptoms of chronic disease, we did not have any clue of malignancy. Thus we palpated the lymph node of neck, axilla and inguinal area everyday. At 27th day after admission, eventually, we found small, 1×1 cm sized, non-tender, fixed mass behind right sternocleidomastoid muscle. By the findings of excisional biopsy of lymph node, we conclude that the underlying cause of pulmonary embolism and deep vein thrombosis of superior vena cava would be large cell lung cancer.
In 1878, Billroth first reported his autopsy observations that human tumor cells were found frequently in association with thrombi12). In the first half of this century, others confirmed the appearance of thrombi in association with viable tumor metastases and primary tumor deposits using conventional histologic sections and light microscopy. More recent immunochemical and ultrastructural studies have confirmed the presence of fibrin deposition in and around both animal and human tumors13). The prevalence of overt or subclinical intravascular coagulation is high in patients with cancer, and up to 92% of all cancer patients have abnormalities on routine blood coagulation tests10,14). Many have found elevated clotting factors in the patients with malignancy, and the commonly elevated factors are Factor I, V, VIII:C, IX and XI15). In addition, increased titer of fibrinogen degradation products (FDP), fibrinopeptide A, cryofibrinogens, fibrin monomer, B-beta 15–42 and related peptides, platelet factor 4, beta-thromboglobulin, and altered fibronectin and antithrombin levels are seen in many patients with disseminated malignancy10,14,16,17). Hypercoagulability in cancer patients may also arise from platelet abnormalities. In addition, a correlation was noted between platelet adhesiveness and the development of thrombosis; the increased platelet adhesion was better correlated with thrombosis than was thrombocytosis in malignancy17,18). In bronchogenic carcinoma, hyperfibrinogenemia and thrombocytosis are found to be elevated in 82% and 30% among 50 advanced cases, respectively19). When frequency of pulmonary embolism at autopsy is determined for various types of cancer, the overall incidence of clinical thromboembolic disease in patients with cancer has been reported to vary between 1% and 11%20,21).
The tumors are almost inevitably located in the periphery of the lung and usually are unrelated to segmental bronchi. In this case, the mass is also found at peripheral lung region. The cytologic examinations of large cell carcinoma showed large nucleoli, lobulated and dispersed nucleoli, and high nueclear-to-cytoplasmic ratio22,23), which correlated with our cytologic findings. On microscopic findings24), large cell carcinomas are amposed of sheets and rests of neoplastic epithelial cells, arranged in lobules. Cells usually have abundant cytoplasm and enlarged nuclei with prominent nucleoli.