Computed Tomographic Correlation with Pituitary Function in Sheehan’s Syndrome
Article information
Abstract
Twenty six patients with Sheehan’s syndrome were studied with high-resolution computed tomography (CT) and the sequential pituitary stimulation test in order to correlate the CT findings of the sella turcica with the pituitary reserve functions. CT revealed 21 completely empty sella (CES), 4 partially empty sella (PES) and 1 normal sella. Panhypopituitarism occurred in 1 of 4 patients with PES and 20 of 21 with CES. One patient showing normal sella had a normal preservation of prolactin (PRL), thyroid stimulating hormone (TSH), follicle stimulating hormone (FSH) and lutenizing hormone (LH). In all patients with PES and CES, growth hormone (GH) responses to hypoglycemia and PRL responses to thyrotropin releasing hormone (TRH) were blunted. Three (75.0%) with PES had normal basal cortisol levels, which were more frequent than two (9.6%) with CES; however, most of the PES (3 of 4) and CES (20 of 21) demonstrated blunted cortisol responses to hypoglycemia. Three (75.0%) with PES and only one (4.8%) with CES had normal thyroxine levels and TSH responses to TRH. None with PES showed decreased basal and stimulated levels of FSH and LH, whereas 15 of 21 with CES did. The pituitary functions of the patients having considerable amounts of pituitary remnants visualized by CT were relatively preserved for TSH, cortisol, FSH and LH. Considering the above results, changes in the amounts of pituitary remnants detected by CT might correlate with hormonal secretory capacity.
INTRODUCTION
Sheehan’s syndrome, or postpartum pituitary necrosis, is usually associated with massive hemorrhage and shock in the postpartum period. Since the original report by Sheehan1) in 1937, the prevalence of Sheehan’s syndrome has decreased in many parts of the world due to improved obstetrical care. However, in the developing countries, it is still a major medical problem. Especially in Korea, where many women had previously delivered babies at home without adequate obstetric care, a number of them have Sheehan’s syndrome.
The onset of symptoms and signs of pituitary hormonal insufficiency in Sheehan’s syndrome varies from patient to patient due to the extent of the infarction and the rate of regeneration of the pituitary2,3). Therefore, it is difficult to predict the time needed to replace deficient hormones, especially cortisol and thyroid hormones which are essential in maintaining life.
CT has emerged as a major noninvasive tool for the diagnosis of intracranial lesions, including those in and around the pituitary4). Coronal sections of pituitary fossa demonstrate that the common findings in Sheehan’s syndrome are varying degrees of emptiness of sella turcica and completely and partially empty sella according to the amounts of the pituitary remnants5).
In this study, we performed high resolution CT and pituitary stimulation tests in order to correlate the emptiness of sella turcica on CT evaluation with the preservation of pituitrary function.
PATIENTS AND METHODS
1. Patients
We examined 26 Korean women with Sheehan’s syndrome, aged 27 to 70, who were admitted to Severance Hospital, Seoul, Korea from 1975 to 1989 (Table 1). Twenty-two patients had histories of postpartum hemorrhage during home delivery without adequate obstetric care. Four (patient 5, 11, 20, 24) of these 22 patients had loss of consciousness due to severe hemorrhage. Four patients (patient 1, 1, 11, 16) received multiple transfusions. Two patients had hysterectomies due to severe hemorrhage during (patient 1) and after (patients 16) labor. All patients failed to lactate after delivery.
2. Methods
CT scans were done in all patients with GE 9800 scanner or Philips tomoscan 310 utilizing a standard protocol that included intravenous infusion of contrast material (150 ml of 60% conray). The slice thickness was 1.5 mm. Coronal scannings were performed perpendicularly to the line from tuberculum sella to dorsum sella, and sagittal reconstruction images were obtained in all patients. Results of CT scans were tabulated by a single radiologist without knowing the individual endocrine and clinical correlation. The presence or degree of the emptiness of sella was determined by the degree of cisternal herniation (modified from Roppolo’s method6)): normal sella up to 50% herniation; PES, 50–90%; CES, more than 90%.
Combined pituitary funciton tests were performed in all patients at the time of CT studies. The schedule of tests was as follows; blood samples were drawn at 30 minute intervals for two hours after combined intravenous injections of 200 ug of synthetic TRH (Hoechst Co.), 100 ug of synthetic LH-RH (Hoechst Co.) and 0.1–0.15 u/kg of regular insulin. Serum was separated and stored at −20′C until assayed. Hormonal levels were measured by radioimmunoassay using commercial kits: GH (Serono Diagnostics, Switzerland); TSH and PRL (Daiabot, Japan); Cortisol (Baxter, USA); FSH and LH (Amersham, UK); thyroxine (Abbott, Chicago, IL).
RESULTS
1. Clinical and Radiological Characteristics
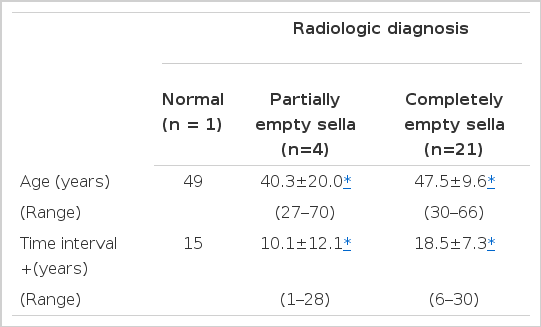
CT scans revealed 21 CES, 4 PES and 1 normal sella. Patient 1 had a normal pitjuitary gland without cisternal herniation. In four patients (paient 2, 3, 4, 5), more pituitary tissue was seen and the sella was partially empty. Three (patients 2, 3, 5) of them showed about 70% cisternal herniation, and one (patients 4) showed 50%. CT scans of patient 6 to 26 revealed the contents of the sella turcica to be primarily of cerebrospinal fluid density, with either no pituitary tissue or a markedly reduced pituitary volume of less than 10%. There were no significant differences in average ages (40.3±20.0, 47.5±9.6 year), and time intervals (10.1±12.1 18.5±7.3 years) between pregnancy related events and CT studies in patients with PES and CES (Table 2).
2. Endocrinologic Studies
In patient 1, normal basal levels of hormones and normal responses of TSH, PRL, FSH, and LH were demonstrated, whereas GH and cortisol responses were blunted. All the patients, except one (patient 6), had basal GH levels of 0.5ng/ml or less. GH level of patient 6 was 0.6 ng/ml. GH responses to insulin hypoglycemia stimulation were blunted in all patients with PES and CES. Basal PRL levels were below the normal range in two of four with PES and nine of 21 with CES. All the patients with PES and CES showed lack of PRL response to TRH administration. While three with PES and two with CES had normal basal cortisol levels, most of the patients with PES (3 of 4) and CES (20 of 21) demonstrated blunted cortisol responses to insulin hypoglycemia stimulation. Only one (patient 4) with PES had a subonormal thyroxine and blunted TSH response to TRH administration, whereas most of the patients with CES showed that basal TSH did not increase inspite of subnormal thyroxine, and TSH responses were also blunted. Basal FSH and LH levels were normal in all patients with PES. Two of them showed adequate FSH and LH responses to LH-RH administration. While 6 and 7 with CES had normal FSH and LH levels, all the CES showed subnormal FSH and LH responses.
As a result of the combined pituitary stimulation test, panhypopituitarism occurred in 21 patients, and twenty of them showed CES. The remaining five patients showed some preservation of pituitary function in TSH, cortisol, FSH, and LH. Four of the five had considerable amounts of pituitary tissue.
DISCUSSION
Acute ischemic necrosis of the pituitary associated with blood loss during delivery was described first by Sheehan in 19371), following considerable research and a number of reports7–9). However, it is regarded as a rare complication in modern obstetrics. A failure of lactation is an early indication that pituitary function has been impaired. Typically, however, the additional clinical features of pituitary insufficiency appear much later. Although the natural history of postpartum pituitary necrosis is unknown, late-onset myxedema and adrenal insufficiency resulting in death have been reported7,10). Therefore, the detection of impairment in endocrine function early in the course of the disease, before gross evidence of endocrine insufficiency develops, is of clinical importance. According to Jialal et al.2), measurement of PRL concentration following challenge with TRH is a convenient and suitable screening test for the detection of Sheehan’s syndrome. However, insulin hypoglycemia test is necessary in order to determine the deficient hormone needed for replacement therapy. Insulin hypoglycemia test is potentially dangerous and impractical for use on an outpatient basis. So, a simple and reliable test is needed to predict the pituitary function.
Computed tomography, one of the modern radiological techniques, has been used for documenting morphologic manifestation of the pituitary gland. Empty sella is the common CT finding of Sheehan’s syndrome when the patients have symptoms of hormonal insufficiency5,11). Sheehan’s studies on women undergoing autopsy within the first month after delivery showed that reduction in pituitary volume did not occur immediately after the pituitary necrosis1). In an early stage of Sheehan’s syndrome, homogeneous or inhomogeneous pituitary tissue might have been detected by CT. In addition, by his autopsy studies on women surviving more than one year, Sheehan observed scarring atrophy and shrinkage of the pituitary gland9). Accordingly, after the necrosis, the pituitary gland shrinks gradually and evolutes to empty sella. Loss of pituitary tissue creates an empty space within the fossa with subsdequent herniation of the arachnoid, entrance of CSF into the sella and appearance of the so-called secondary empty sella on CT scans. Similarily, secondary empty sella occurs after surgical removal12), radiation therapy12) or spontaneous infarction13) of pituitary tumors and represents replacement of tumor mass with fluid. In such patients, the sella is enlarged. however, in Sheehan’s syndrome, the sella is not enlarged. In this study, the time intervals between CT studies and postpartum bleeding were more than 6 years in all cases except one (patient 3), and the results revealed that of the 26 patients one showed normal sella, 4 PES and 21 CES.
The most common endocrine abnormality found in all patients was deficiency in the ability of the pituitary gland which produces GH in response to insulin hypoglycemia and prolactin in response to TRH. It means that the observation of poor GH and prolactin reserve is possibly a result of the anatomic situation of GH and prolactin cells in the lower lateral regions of the adenohypophysis, which are the most susceptible to damage by ischemic necrosis14).
The determination of impairment in the ACTH reserve in patients with Sheehan’s syndrome is of clinical importance because of possible life-threatening cortisol deficiency. So, it is necessary to study ACTH reserve under stress. Although the insulin induced hypoglycemia is a specific stress test, its relationship to other stressful situations, such as a surgical procedure, has not been proved. Hypoglycemia followed by an abnormal cortisol response is a reflection of poor ACTH reserve15). Deaths due to adrenal insufficiency in patients with postpartum hypopituitarism have been reported previously7,10). Consequently, it is recommended that the patients with Sheehan’s syndrome showing a deficient cortisol response to hypoglycemia should be treated with cortisol replacement15).
Three (75%) with PES had normal basal cortisol levels, more frequent than 2(9.6%) with CES. This result indicates that the reduction in pituitary ACTH reserve capacity was more severe in CES than in PES. However, most of the patients with PES and CES demonstrated blunted cortisol responses to hypoglycemic stimulation. Therefore, cortisol replacement therapies are needed.
According to Dizerega et al15), use of thyroid hormone replacement should be dependent upon the free thyroxine index, not on the TSH response to TRH administration. The fact that most of the patients with CES had low thyroxine levels and blunted TSH responses to TRH administration indicates the need for thyroid hormone replacement.
It is interesting that normal thyroxine levels were observed in 3 of 4 with PES. The TSH reserve in the pituitary of the patients with PES appeared sufficient to maintain normal thyroid funciton without clinical symptoms of hypothyroidism. Accordingly these patients do not, at present, need thyroid hormone replacement. T4 and FT4 should be measured twice a year in order to detect deterioration and determine subsequent for therapy7).
Basal gonadotrophin levels were normal in all patients with PES and gonadotropin responses to LH-RH administration were adequate in 2 of 4 with PES. These results indicate that considerable numbers with PES have the pituitary reserve-function necessary to maintain the normal basal gonadotropin and to release gonadotropin in response to gonadotropin releasing hormone.
Sheehan16) reported that, even in the presence of massive necrosis, there is some residual tissue at the pars tuberalis and the lateral poles of the pituitary gland. Therefore, those areas are able to respond to exogenous gonadotropin releasing hormone. So, it is anticipated that gonadotropin reserve function is preserved more or lee even in those patients with CES who have the pituitary volume less than 10%. Contrary to Sheehan’s report, however, only one of 21 with CES showed normal gonadotropin response to the administration of the gonadotropin releasing hormone.
Fleckman et al.5) had found no correlation between the presence of pituitary tissue in CT evaluation and preservation of the function in the study on 13 patients with Sheehan’s syndrome. In their study, some preservation of pituitary function was observed not only with PES but also in considerable numbers with CES. In our study, panhypopituitarisms occurred in 21 patients. Twenty of them showed CES. The remaining five patients had some preservation of pituitary function. Only one (patient 9) of the five was revealed to have CES. The pituitary function of the patients having considerable amounts of pituitary remnants visualized by CT were relatively preserved for TSH, cortisol, FSH and LH. Considering the above results, it might be predicted that the changes in the amounts of pituitary remnants detected by CT correlate with hormonal secretory capacity in patients with Sheehan’s syndrome who had a history of postpartum massive bleeding. This result contrasts to that of Fleckman’s study. However, it will be necessary to study further the numbers with PES in order to detect deficient hormone according to the region of remaining tissue. Also, the continuous observation of changes in the pituitary function and remaining tissue in patients with PES is required for defining the natural course of Sheehan’s syndrome.
It is noteworthy that patient 4 showed a panhypopituitarism in spite of the remaining pituitary tissue occupying 50% of sella turcica. She was 70 years old, and the age at the postpartum bleeding was 42. We thought her hypopituitarism developed due to senility or the long interval between CT study and postpartum bleeding. However, her pituitary remnant was possibly composed of mainly scarred tissue.
In summary, CT scannings in patients with Sheehan’s syndrome revealed 21 CES, 4 PES and 1 normal. Most of the patients with CES showed panhypopituitarisms. The pituitary reserve capacity for TSH, cortisol, FSH and LH was preserved more or less in patients having considerable amounts of pituitary remnants visualized by CT. We recommend sella CT for the patient with Sheehan’s syndrome in order to determine the degree of hormonal insufficiency.