Characteristics of Anti-TSH antibody and Its Relationship with TSH Receptor Antibody*
Article information
Abstract
Using the TSH binding inhibition IgG (TBII) assay three patients with Graves’ disease were discovered to have serum TSH-binding immunoglobulins of high affinity. These IgGs bound 61%, 33% and 60% of radiolabeled TSH, respectively, higher than the maximal specific binding (25%) in the TBII assay. Such binding was detected even in the absence of TSH receptor with only small differences in the precipitable radioactivity (61 %, 28%, and 61 %, respectively) compared with non-specific binding (11.3%). The 125I-bTSH binding of IgGs was competitively inhibited by the addition of bTSH. The 7s fraction was found to be a major binding component by gel filtration chromatography. Myxedema sera with high TSH levels did not affect the reaction. Moreover IgG binding to bTSH was not inhibited by the addition of serial dilutions of TBII positive pooled Graves’ IgG (0.1–10mg/ml) from a different untreated patient. The titers of these TSH binding antibodies were not changed during the treatment of Graves’ disease. Following guinea pig fat cell membrane receptor purification, the IgG of one patient with Graves’ disease revealed TBII activity of 46.3%. However, no binding of 125I-bTSH in the absence of the TSH receptor was evident.
These studies suggest that 1) anti-TSH antibodies and TSH receptor antibodies are present independent of one another in the sera of some patients with Graves’ disease, and 2) TSH receptor antibodies do not affect the binding of anti-TSH antibodies to TSH.
INTRODUCTION
It has been reported that TSH binding immunoglobulins are present in the sera of some patients with Graves’ disease.1–6) However, the mechanism of their formation is not known. The biological roles of these antibodies such as their relationship with TSH receptor antibodies is also uncertain. Recently Biro2) and Raines et al6) suggested that anti-TSH antibodies could be formed as anti-idiotype antibodies to TSH receptor antibodies present in Graves’ sera. This possibility is supported by the findings of inhibition of TSH receptor antibody binding to TSH receptors by anti-TSH antibodies and inhibition of anti-TSH antibody binding to TSH by Graves’ IgG.6) If anti-TSH antibodies are anti-idiotype, one might expect the clinical course of Graves’ disease to be affected and the titers of these antibodies to be altered during the treatment of Graves’ disease.
In the present study, we documented the presence of anti-TSH antibodies in patients with Graves’ disease. We observed that the titers of these antibodies were not changed during the treatment of Graves’ disease. Both TSH receptor antibodies and anti-TSH antibodies were present independent of one another in the sera of patients with Graves’ disease and TSH receptor antibodies did not affect the binding of anti-TSH antibodies to TSH.
MATERIALS AND METHODS
1. Patients
Patient 1. A 62 year-old man was admitted to Seoul National University Hospital (on April 10, 1985) because of weight loss and arthralgia. He had lost 13kg of weight during the previous 2 years and arthralgia had persisted for 7 months before admission. Physical examination revealed tremor of the hand, moist skin and proximal muscle wasting. Neither goiter nor exophthalmos was present. As shown in Table 1, laboratory findings indicated a diagnosis of hyperthyroidism. He was treated with methimazole and atenolol. He had never received exogenous TSH.
Patient 2. A 38 year-old man was first diagnosed as having hyperthyroid Graves’ disease at the age of 35 years. He was treated with methimazole from 1982 to May, 1984. For 3 months he had suffered from heat intolerance, hyperhydrosis, palpitations and weight loss of 3kg. His brother had also been treated for Graves’ hyperthyroidism. Physical examination revealed tachycardia (100/min), tremor of the hands, warm moist skin, a moderate-sized diffuse goiter (about 50g), and mild proptosis with lid retraction. As shown in Table 1, laboratory findings indicated a diagnosis of hyperthryoidism. He was treated with 10 mCi of 131I and methimazole. He had never received exogenous TSH.
Patient 3. A 70 year-old woman was diagnosed as having Graves’ hyperthyroidism and was started on treatment with methimazole. At the time of the present study she was euthyroid while receiving 5mg methimazole daily. A firm diffuse goiter of moderate size (about 60g) was present. She had never received exogenous TSH.
2. Preparation of IgG Fraction
The IgG fractions from sera were prepared by means of affinity chromatography on columns of protein A-Sepharose CL-4B (Pharamica, Sweden). The protein concentrations were determined by the method of Lowry and co-workers.7)
3. TBII Assay
TBII activity was measured using the TSH receptor antibody kit prepared by R.S.R. Ltd. (Cardiff, Wales United Kingdom). The serum (50μl) or IgG (100 μl of, 10mg/ml) was incubated with solubilized porcine thyroid membrane (50μl). After 15 min at room temperature, 125I-bTSH (100μl, 10,000 cpm) was added, and the mixture was incubated for 60min at 37°C. 30% PEG containing 1 M NaCl was then added to make the final concentration 15% (wt/vol). After centrifugation at 1500 × g for 30 min, the radioactivity of the pellet was counted. TBII was calculated according to the following formula:
The Normal range of TBII in 69 normal subjects was from −10 to 14.9%. A TBII value exceeding 15% was considered abnormal or positive
4. Binding of 125I-bTSH by IgG: Displacement by bTSH
Thytropar (Amour, Phoenix, AZ, USA) in various concentrations (1μU-10mU/ml) in 10mM Tris/50mM NaCl/0.1 % BSA, pH 7.4 (100μl) was added to the mixtures of IgG (100μl, 10mg/ml) with 125I-bTSH (100μl). After 1 h at 37°C, the bound fractions were precipitated with cold PEG [final concentration, 15% (wt/vl)]. Radioactivity in the pellets was then measured. Scatchard analysis was performed by assuming that pure TSH has a potency of 40U/mg and a molecular weight of 28,000
5. Effect of Sera Containing High TSH and TBII Concentrations on 125I-bTSH Binding
Three patients with primary nongoitrous myxedema (patients A, B and C) served as sources (serum TSH values 90, 70 and 110μU/ml, respectively). Fifty microliters of serum were added to a mixture of 100μl patient 1 IgG (10mg/ml) and 100μl 125I-bTSH. An equal quantity of normal serum was used as a control. Incubation and PEG precipitation were performed as described above (TBII assay).
TBII-positive pooled Graves’-IgGs were prepared from 7 patients with untreated Graves’ disease. The TBII value of the pooled Graves’-IgG was 70%. Ten mg/ml of IgGs from each patient with anti-TSH antibodies were incubated with different concentrations, from 0.1 to 10 mg/ml, of pooled Graves’-IgG for 60min at 37°C before adding 100μl 125I-bTSH. An equal quantity of normal pooled IgG was used as control. Incubations and PEG precipitation were performed as described above (TBII assay).
6. Heat Treatment of IgG and TSH Receptor
Patient 1 IgG and TSH receptor were heated in a water bath for 30 min at 56°C. 125I-bTSH binding to patient’s IgG and TSH receptor were performed as described above (TBII assay).
7. Gel Filtration Chromatography
A 2 × 100cm column of Sephadex G-200 (Pharmacia, Sweden) was prepared. A 750μl serum sample was applied, and elution was performed with PBS, pH 7.4. Consecutive fractions (2ml) were collected and their OD’s determined. To 800μl of each fraction, 100μl 125I-bTSH was added and mixtures were incubated for 1 h at 37°C. 100μl of pooled normal IgG (10mg/ml) was then added, and IgG precipitated with cold 30% PEG containing 1 M NaCl. After centrifugation, the radioactivity of each pellet was counted.
8. Receptor Purification of TBII from Graves’ IgG
Guinea pig fat cell membranes (28mg protein) were incubated with 4mg/ml IgG (patient 1) in a total volume of 8ml 10mM Tris/50mM NaCl/0.1 % BSA buffer, pH. 7.4 for 60 min at 37°C.8) At the end of incubation, the suspension was centrifuged at 15,000 × g for 20 min at 4°C. The pellet was washed three times with buffer and then resuspended, in 8ml 0.1 M glycine-HCl buffer, pH 3.0 for 60min at 4°C. Membranes were again sedimented, and the supernatant, which contained eluted TBII, was collected. The supernatant was brought to pH 7.4 with 1 N NaOH, concentrated, and then the buffer was changed to 10mM Tris/50mM NaCl, pH 7.4. The final supernatant was centrifuged at 2000 × g for 20 min at 4°C to remove denatured protein. TBII activity was determined by the methods described above. One mg/ml protein concentration of receptor purified IgG was used for the determination of TBII activity.
RESULTS
1. TBII Assay
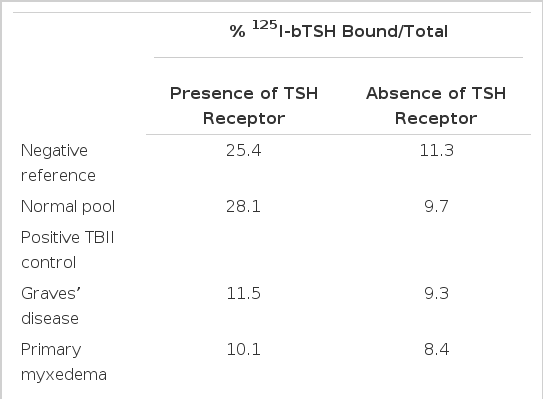
As show in Table 2, negative reference and normal pooled sera showed 25.4% and 28.1 % specific binding of 125I-bTSH to the TSH receptor, respectively. Nonspecific binding, measured by substitution of 1 % Lubrol solution for the TSH receptor, was about 10%. Specific binding of 125I-bTSH to the TSH receptor was displaced effectively by the TBII positive Graves’ and primary myxedema (blocking type TBII) sera to a level close to that of nonspecific binding. Sera from patients 1, 2 and 3 gave unexpectedly high PEG-precipitable radioactivities of 61.4%, 33.1% and 59.9%, respectively, in the presence of the TSH receptor. However, in contrast with the results of normal pooled and TBII positive sera, only small differences (60.9%, 28.2% and 60.0%, respectively) were found in the absence of the TSH receptor. Preparations of IgG separated using protein A-Sepharose CL-4B affinity chromatography gave similar results. Progressive dilution of IgGs yielded a progressive reduction of PEG-precipitable radioactivities in the absence of the TSH receptor (data not shown).
2. Effects of TSH and TSH Receptor Antibodies
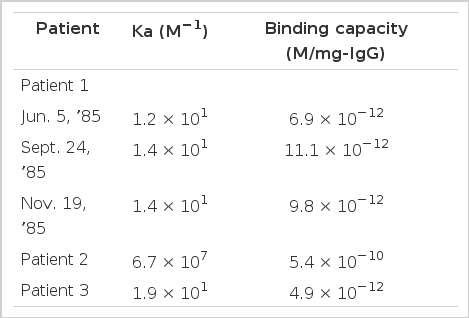
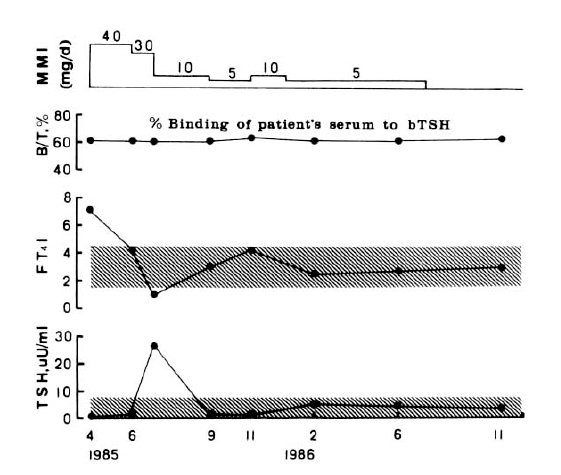
Addition of unlabelled bTSH produced a concentration-dependent inhibition of the binding of 125I-bTSH by IgGs of all three patients. Scatchand analysis revealed that the IgG of patient 1 bound 125I-bTSH with an association constant of 1.2 × 1010 M−1 and a maximum binding capacity of 6.9 × 10−12M/mg IgG. The binding affinity did not change during antithyroid drug treatment (Fig. 1). Association constants of patients 2 and 3 IgGs were 6.7 × 107 M−1 1.9 × 1010 M−1, respectively, and maximum binding capacities were 5.4 × 10−10, 4.9 × 10−2M/mg IgG, respectively (Table 3).

Displacement of PEG-precipitable 125I-bTSH binding by IgGs from patient 1 by bTSH. Incubation was carried out in 100 μl IgG (10mg/ml) and 100μl 125I-bTSH in addition to 100 μl of 10mM Tris/50mM NaCl/0.1% BSA, pH 7.4, containing 1–10 μU/ml bTSH. The insert shows the Scatchard plot of bTSH binding to patient’s IgGs.
None of the three sera with high TSH concentrations (70–110μU/ml) significantly inhibited 125I-bTSH binding to the IgG of patient 1 (Fig. 2).
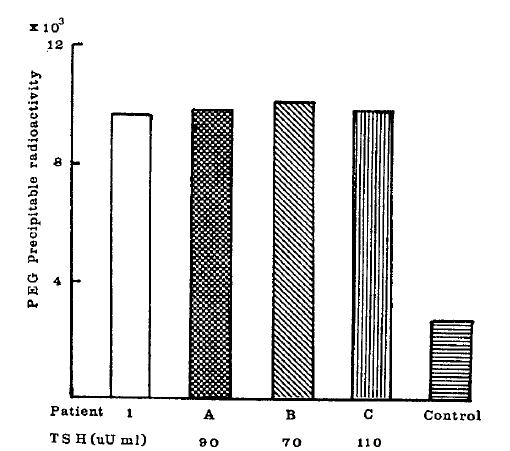
Effects of sera from patients (A, B, C) with high TSH on PEG precipitable 125I-bTSH when incubated with patient 1 IgG. For comparison, nonspecific binding by TBII negative reference IgG (□) and by patient 1 IgG (□) are shown.
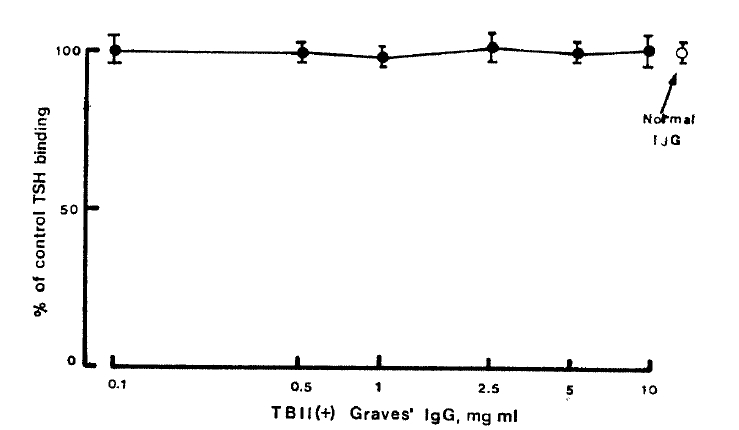
To evaluate the effect of TSH receptor antibodies on the125I-bTSH binding by the patients’ IgGs, the patients’ IgGs were incubated with TBII-positive pooled Graves’ IgG before assay for TSH binding. As shown in Figure 3, TSH receptor antibodies, at concentrations up to 10mg/ml, did not affect the binding of 125I-bTSH to the patients’ IgGs.
3. Effect of Heat Treatment
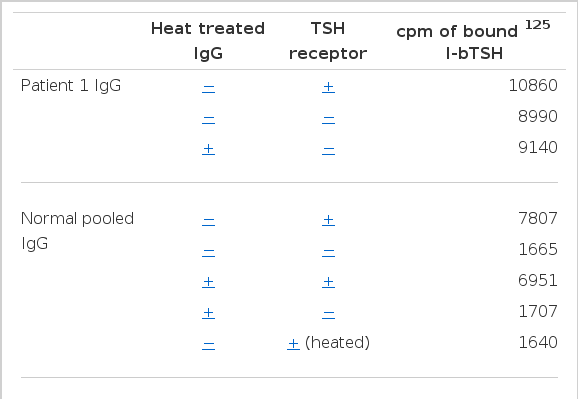
Since circulating immune complexes formed between the TSH receptor and receptor antibody might be responsible for the abnormal 125I-bTSH binding, the effect of heat treatment was studied. As shown in Table 4, the 125I-bTSH radioactivity precipitable with IgG from patient 1 was not affected by heat treatment. Neither specific binding of normal IgG nor the displacing ability of TBII-positive IgG was affected by heating. On the other hand, heating of the TSH receptor completely abolished its TSH-binding capacity.
4. Gel Filtration Chromatography
Sera from patients 1 and 2 were subjected to gel chromatography on Sephadex G-200. 125I-bTSH and normal IgG were added to each fraction and the radioactivity precipitable by PEG in each was determined. As shown in Figure 4, peak bTSH binding activity in both patients’ sera coincided with the 7S IgG fractions.
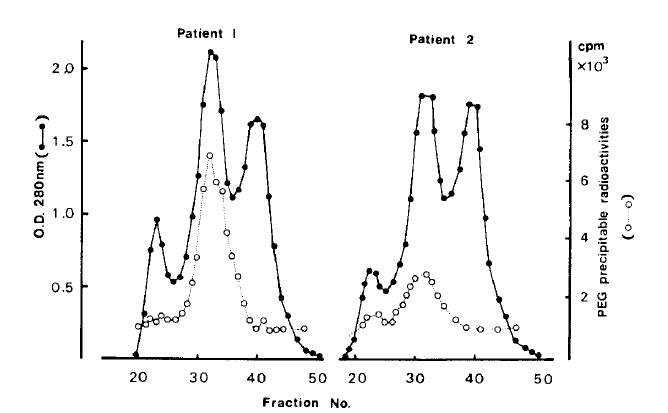
125I-bTSH binding activity of patient 1 (left) and patient 2 (right) serum fractions. Sera from both patients were applied to a Sephadex G-200 column and eluted with PBS. Optical density (●–––––●)was read at 280 nm by spectrophotometry. Each fraction was incubated with 125I-bTSH, and then precipitated with PEG. The radioactivities of the pellet were counted (○···· ○)
5. TBII activity in receptor purified IgG
To asses the TBII activity in IgGs which revealed abnormal binding of 125I-bTSH, we prepared receptor purified IgG from Graves’ patient 1 using guinea pig fat cell membranes and performed TBII assays. As shown in table 5, whole IgG which is not receptor purified revealed abnormally high binding of 125I-bTSH in the presence and absence of the receptor. However, receptor purified IgG showed specific inhibition of 125I-bTSH binding to the TSH receptor (TBII = 46.3%). In the absence of the TSH receptor, non-specific binding of 125I-bTSH was similar to that of normal pooled IgG.
6. Changes in the Titers of anti-TSH Antibodies During Treatment
Figure 5 shows serial changes of thyroid function and percent binding of 125I-bTSH the serum of patient 1 during antithyroid drug treatment. Although all clinical and laboratory findings were normalized by antithyroid drug treatment, the titers of bTSH binding activities were not changed during 19 months. Anti-TSH antibody did not affect the measurement of serum TSH by radioimmunoassay using double antibody separation. The titers of bTSH binding activities were also not changed in the two other patients with Graves’ disease.
DISCUSSION
In the present study, we demonstrated that IgGs from patients with Graves’ disease bound 125I-bTSH when tested in the TBII assay. Binding of bTSH in these sera was consistently seen and persisted for months in all patients. The 7s IgG fraction was found to be the major binding component by gel filtration chromatography. Binding of 125I-bTSH by IgGs was inhibited dose-dependently by unlabelled bTSH but was not inhibited by myxedema sera containing high TSH concentrations (70–110 μU/ml). It was decreased dose-dependently by serial dilutions, however, heat treatment did not alter the binding property of this IgG, while the binding ability of TSH receptor was almost completely lost. None of the patients had previously received exogenous TSH. Therefore, these studies suggest that these three patients had spontaneously developed anti-TSH antibodies which bind bTSH but not hTSH. Our results concerning anti-TSH antibodies are consonant with the reports of several investigators,1–6) who also described the presence of TSH-binding immunoglobulins in the sera of some patients with Graves’ disease.
There are several hypotheses to explain the mechanism of production of these anti-TSH antibodies, however, none of them have been proven. Firstly, these TSH-binding immunoglobulins could be antibodies formed against exogenous TSH or TSH-like substances to which the patients may have been exposed. This theory is unlikely because all reported patients including our cases had never received exogenous TSH. Secondly, anti-TSH antibodies could be formed as a reaction against endogenous human TSH.9) In Graves’ disease, formation of anti-TSH antibodies as the primary immunological response might then evoke the generation of anti-idiotypic antibodies capable of binding to the TSH receptor with resulting hyperthyroidism. This theory is supported partly by experimental results. Islam et al9) injected rat antihuman TSH antibodies into rabbits and generated antibody-stimulated thyroid adenylate cyclase activity and iodide transport.
If TSH receptor antibodies were anti-idiotypic antibodies against anti-TSH antibodies, anti-TSH antibodies should be formed as a primary immunological event in all patients with Graves’ disease. However only a small proportion of patients with Graves’ disease have anti-TSH antibodies. Kajita et al4) found TSH-binding antibodies in only 2 of 102 patients with Graves’ disease and Akamizu et al5) reported that 2 of 154 patients with Graves’ disease had anti-TSH antibodies. We found anti-TSH antibodies in only 3 of 500 patients with Graves’ disease. Therefore this sequence of events is unlikely.
A third possibility is that anti-TSH antibodies are formed as anti-idiotypic antibodies against TSH-receptor antibodies. This hypothesis is attractive because TSH receptor antibodies are detected in the sera of almost all patients with Graves’ disease,10–12) consistent with a primary immune response against the TSH receptor. Recently Raines et al6) reported that TSH binding of TSH-binding antibodies was inhibited by Graves’ IgG and TSH-binding antibodies inhibited TSH receptor antibodies binding to the TSH receptor. They suggested that TSH-binding antibodies may represent anti-idiotypic antibodies. However, as in the studies of Akamizu et al,5) we could not document any direct effect of TSH receptor antibodies on TSH binding of anti-TSH antibodies. The titers of anti-TSH antibodies in patients with Graves’ disease were not changed for several months, even though all clinical and laboratory findings were normalized during antithyroid and radioactive iodine treatments.
Furthermore we could separate the TSH receptor antibodies from anti-TSH antibodies by receptor purification using guinea pig fat cell membranes. Receptor purified IgG produced specific inhibition of 125I-bTSH binding to the TSH receptor but did not show any 125I-bTSH binding activities. This finding suggests that anti-TSH antibodies and TSH receptor antibodies are present independent of one another in the sera of some patients with Graves’ disease.
If anti-TSH antibodies were anti-idiotypes to TSH receptor antibodies, anti-TSH antibodies may be expected to bind directly to TSH receptor antibodies rendering them incapable of binding to the TSH receptor and thus modulating the clinical course of Graves’ disease. However, in the present study, we could not demonstrate any interaction between anti-TSH antibodies and TSH receptor antibodies or show any effect of anti-TSH antibodies on the clinical course of Graves’ disease. Moreover, these anti-TSH antibodies did not react to hTSH and did not affect the measurement of serum TSH in our patients. Although the mechanism of production of the antibody for bTSH is unknown, it may be produced against a common portion of TSH protein in various non-human species,4) or as a result of a conformational change in the hTSH molecule which might occur due to some unknown mechanism in patients with autoimmune thyroid diseases.
Acknowledgements
The authors are indebted to Dr. Sidney H. Ingbar for his helpful reviews and discussion of the manuscript, to Mr. Jae Min Chung and Ms. Kyung Sun Min for their technical assistance, and to Ms. Eun Ja Kwack and Ms. Jung Hee Choi who skillfully prepared the manuscript.