Isoextraction Studies of Partition of UCB Between Chloroform and Aqueous Solution
Article information
Abstract
Bilirubin is a major component of gallstones and the solubility of unconjugated bilirubin (UCB) has been shown to play an Important role in gallstone formation. The mechanism of gallstone formation and solubilization of UCB is, however, still unknown.
Several investigators have attempted to determine the chemical nature of bilirubin which are thought to be closely related to its solubility. However, there is some controversy over the ionization constants and pK values of carboxyl groups of UCB.
In the present study, the effect of pH, the type and concentration of bile salt on UCB solubility were studied and the pK values of bilirubin were calculated.
The first ionization censtants of the carboxyl group of UCB (pk1) were 7.2 in saline, 6.7 in TC (Taurocholic acid), and 6.6 in TDHC (Taurodehydrocholic acid).
The pK2 was 9.2 in 50 m M TC. Thus, UCB monoanion (BH−) accounted for over 90% of the ionized UCB at physiologic pH values.
Our results suggest that:
With the isoextraction method of UCB from chloroform, crystal surface effects are avoided and the partition equilibrium could also be attained rapidly.
There are more solubilities with higher bile salt concentrations and with increasing pH, and bile salt plays an important role in solubilizing UCB in bile.
Bile salt monomers, dimers and micelles are equally effective in solubilizing bilirubin.
The bilirubin in bile is almost entirely in the monoanion (BH−) form at physiological pH values.
INTRODUCTION
The authors examined the partition of UCB (Unconjugated Bilirubin) between chloroform (CHCl3) and aqueous solution by isoextraction. The purpose of this study is to determine the interaction of UCB with bile salt and determine pK values for UCB in bile salt solution. Several investigators have attempted to determine the dissociation constants of bilirubin which are closely related to its solubility. But there have been some problems; a) bilirubin has a property of being decomposed easily under oxygen & it is extremely photosensitive (configurational isomerization, oxidation) b) to determine the correct value is difficult because of the extremely small solubility product of bilirubin1–14).
Bilirubin is a major component of gallstone5–9) and the aqueous solubility of UCB has been shown to play an important role in gall stone formation10–11). However, the mechanism of gallstone formation and solubilization of UCB is still unclear. UCB is extremely insoluble in water near physiological pH values12–13) and this poor aqueous solubility of UCB is considered to reflect a molecular conformation in which all the hydrophilic regions of the molecule were involved in intramolecular hydrogen bonding14–15).
In UCB with fully protonated form (BH2), each of the 2 carboxyl groups forms intramolecular hydogen bonds, which stabilize the bilirubin molecule in a rigid conformation, suppress the ionization of the carboxyl groups and monopolize all of the polar groups in the bilirubin molecule15–16). As a result, crystalline UCB dissolves poorly in buffered aqueous solutions at physiological pH values, and has an apparent pK value in the weakly alkaline range2).
Theoretically, bilirubin can exist as a fully protonated diacid (BH2), as a monoanion (BH−) or as a dianion (B=). If bilirubin is equilibrated with a buffer, it forms a saturated solution containing a very low concentration of the acid and a higher concentration of the dianion. The total concentration can then be measured and when the dissociation constants are known, the concentration of the acid, which is equal to its solubility, can be calculated. However, investigators have not been able to agree as to the ionization constants of the carboxyl groups or even whether the 2 groups have identical or different pK values17–19). The controversy over the pK values is that intramolecular hydrogen bonds could stabilize the bilirubin acid & then shift the pK to higher values.
The authors have studied the maximum “equilibrium” solubilization of UCB crystal in bile salt solution but could not be certain that true equilibrium had been reached because the surface effects and the structural variations of UCB crystals influenced the crystal dissolution rates, and degradation of UCB could not be completely eliminated. Using isoextraction of bilirubin from chloroform into a buffered solution, these crystal effects were avoided by dispersing the UCB in organic solutions before partition and partition equilibrium were attained rapidly.
The effect of pH, the type and concentration of bile salt on UCB solubility were studied and the pK values of bilirubin were calculated.
MATERIALS AND METHODS
Bilirubin was obtained from Gallard Schlesinger, N.Y. and purified according to McDonagh method20). Organic solvents (chloroform and CCl4) were purified by distilling to remove 0.5% ethanol which is added as a stabilizer, washing with 0.1M NaOH and 0.1M sulfuric acid followed by deionized, charcoal-treated water several times to remove the chemicals which are preservatives and trace contaminants, and then stored in brown bottles, in the dark, under a layer of distilled water for 2 days before use.
Hydrophobic impurities of bile salt (eq.; lipids and unconjugated bile salts) were also removed from the stock bile salt solution by washing with CHCl3 or CCl4 before use.
Isoextraction studies were done with a common lower phase, of 90 or 250 mg bilirubin in 300 ml chloroform in a specially designed vessel with 10 chimneys (Fig. 1), which had been cleaned between experiments by rinsing with chlorofrm, methanol, water, alkali, acid and then, water. Any polar contaminants which could be present in the initial UCB crystals were also removed by prewashing the CHCl3 solution of UCB with water before isoextraction. The pH ranges were from 5.0–9.5 and the aqueous phase (3 ml) had a total ionic strength 0.15 M and contained bile salt and buffer. Buffers used were citrate from pH 5.0–6.0, phosphate from pH 6.5–8.0 and borate from pH 8.5–9.5.
Full range studies were done at 20 and 50 mM Na taurocholate (TC) and with no bile salt; added studies were done at 5 and 10 mM taurocholate for the pH ranges from 6.0–10.0. Studies with taurodehydroxycholate (TDHC) were also performed to compare the solubilization effect of nonmicellar bile salt.
For studies with 10-chimney isoextraction chamber, the solutions were maintained at 25°C, and stirred with an internal Teflon-coated steel bar over a rotating magnet. Oxidation of UCB was minimized by gassing the solutions with nitrogen and keeping the vessel in the dark. Then, absorbance (Optical Density: O.D.) at 453 nm was determined every 24 hours for 4 days, and it was found that equilibrium was attained at 48 hours. So finally, the O.D. of the aqueous phase was measured at 48 and 72 hours, and the partition ratio between the upper and lower phases (P) was calculated from the diazo-assay of UCB21) in both phases at 72 hours.
The stability of UCB was demonstrated by the fact that the addition of 5 mM EDTA as a stabilizer didn’t influence the result. Further evidence of the stability of UCB was obtained by extraction & thin layer chromatography (T.L.C.) of the aqueous phase pigment20,22). (T.L.C. of the equilibrated phases revealed < 2% III α and XIII α isomers and < 1% degradation of bilirubin.
RESULTS
1. Effect of the Duration of Incubation and Total Ionic Strength on UCB Solubility (Fig. 2)
The bilirubin concentration in the upper aqueous phase was stable after 48 hours. Initially, absorbance at 453 nm was determined every 24 hours for 4 days but the O.D. values were constant from 48 hours and on.
Therefore, analysis in definitive studies were performed after 48 hours of incubation. The pH values shown at 48 hours are the initial values of the solutions, and the pH values shown at 72 hours are the final, measured values. The 2 curves are virtually superimposable and all of the curves at 48 and 72 hours were always along the same regression lines. The changes between 48 and 72 hours were due to the shift in pH, not to the degradation of UCB. There were no significant differences in UCB solubility at total ionic strengths from 0.10 to 0.30 M at any pH values.
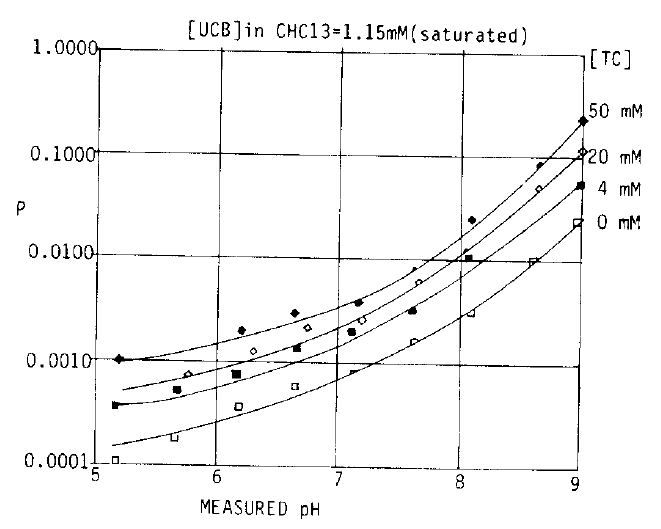
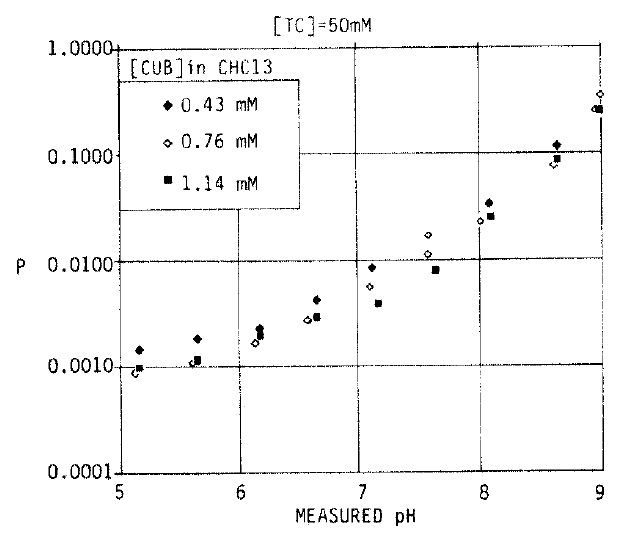
2. Effect of pH and the Amount of Bilirubin on Solubility (Fig. 3)
Effect of [bilirubin] in chloroform phase. Partition is similar over wide range of UCB concentrations, suggesting little self-association of UCB.
Fig. 3 shows the partition ratio between aqueous and organic phases in 50 mM TC in relation to pH. P means partition ratio between aqueous and organic phases and the pH values are final, measured values. The partition ratio was increased linearly with increasing pH, showing that increasing pH values increased the solubility of UCB in buffered aqueous solutions.
This indicates that salts of the monoanion (BH−) and dianion (B=) of UCB are more soluble than the diacid (BH2). This figure also shows that the partition ratio was similar over wide range of bilirubin concentrations in the organic phase at each concentration. Suggesting that there was little self-association of UCB and it was found that the saturation could be achieved with about 1.1 mM UCB in CHCl3.
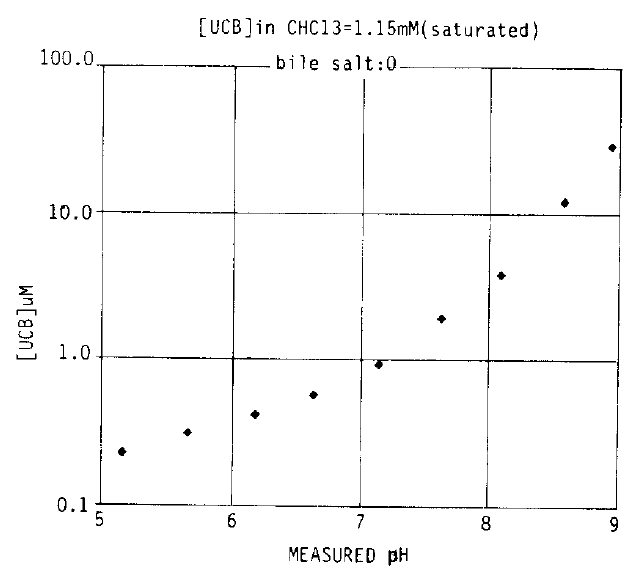
3. Solubility of UCB in Buffered Sodium Chloride Solution (Fig. 4)
In the absence of bile salt, the solubility of UCB was similar to that from dissolution of UCB crystals with a similar slope of curve. Total bilirubin concentration could not be measurable at pH values below 5.0 because there was not sufficient bilirubin in the aqueous phase to give a diazo reaction and this probably reflects the range at which bilirubin is fully protonated in the diacid form (BH2). Total bilirubin concentrations were 0.81 μM at pH 7.14, 3.49 μM at pH 8.09 and 28.68 μM at pH 8.95, but rose steeply at pH ≥9.0 with a steeper increase in the slope of curve, and it may be related to the changes in the molecular state of UCB due to the ionization of CONH groups on one of the end pyrrole rings.
4. Effect of the Type and Concentration of Bile Salts on the Solubility (Fig. 5,6)

Effect of taurodehydrocholate in aqueous phase. Partition increases with increasing [bile salt]. Values are similar to taurocholate.
Increasing bile salt concentration augmented UCB solubility in aqueous solution at all pH ranges, suggesting that all 3 forms of UCB were capable of interaction with bile salts, o and 4 mM TC are below the critical micellar values and 20 and 50 mM TC are above the critical micellar values. Studies with non-micellar bile salt, TDHC were also done to compare the solubilization effect with micellar bile salt, TC and the effect of TC was similar to TDHC. Bilirubin interactions were almost the same in TC and TDHC at each concentration.
Solubilization of UCB by other bile salts, GC (glycocholate) and TCDC (taurochenodeoxycholate) were also almost the same as by TC at pH values of physiologic range of bile. The unconjugated bile salt was not studied because of its limited solubility23).
5. Effect of Bilirubin Purity on Solubility (Fig. 7)

Effect of bilirubin purity on partition. Impurities increase partition into aqueous phase, especially at pH values ≤ 8.0.
It was found that bilirubin purity had an important effect on UCB solubility. Different lots of bilirubin yielded different results and impurities in the bilirubin increased its partition into the aqueous phase, especially at pH values ≤8.0. The shape of the curve and the apparent pK values were also altered. Bilirubin was purified by the Mc Donagh method and tested by T.L.C. on silica gel.
6. Bilirubin Saturation in the Aqueous Phase (Fig. 8, Table 1)
The aqueous solubility (S) curves in Fig. 7 fit the equation:
Table 1 shows the values at saturation yielded by computer analysis of these curves. The apparent pK value for ionization of the first COOH group of bilirubin was 7.2 in the absence of bile salt and the pK values were 6.7 in 50 mM TC and 6.6 in 50 mM TDHC. Since the measured UCB concentrations at pH ≤ 4.0 were too low to be reliable in buffered saline solutions, S0 values had to be estimated by extrapolation. The solubilities in the bile salt solution at pH 8.0 were above the maximum concentrations of UCB found in normal human hepatic bile at comparable bile salt concentrations. All other values were below that level.
Second ionization was not apparent at pH 6.0–8.0 which is physiologic for bile likely due to the internal hydrogen bonding in UCB. Therefore bilirubin in bile was almost entirely in the monoanion (BH−) form.
DISCUSSION
UCB is a major component of the pigment gallstone in the form of Ca bilirubinate or a black pigment polymer24–26), and it is found in normal human gallbladder bile in concentrations up to 35 μM (2 mg/dl)27), even though UCB is insoluble in the buffered aqueous solution12,13).
Concentrations and proportions of UCB appeared to be elevated in the bile of many patients5–8) or rats28). It has been suggested that UCB may be solubilized by the lipid component of bile, esp. bile salts, and that UCB in relation to bile salt might be a factor in the pathogenesis of pigment gallstones29,30). There had been a number of prior studies of the solubility of UCB with or without bile salts3,12,13,31,32) and the authors also studied the solubility of UCB crystals in bile salt solutions.
There were 2 major problems in the equilibrium solubilization of UCB crystals; a) the crystal surface effect influenced the crystal dissolution rate b) degradation of UCB was not completely eliminated, which was evidenced by the development of green discoloration, due to the formation of mesobiliverdin by the internal prototropy of UCB33). Mesobiliverdin could alter the equilibrium solubility of UCB by interaction with bile salts.
To solve these problems, we used the isoextraction method using a 10 chimney-isoextraction chamber (Fig. 1).
Isoextraction is an equilibrium method, the principle is to determine the partition equilibrium of cation and anion between the aqueous and organic phases and it is based on the distribution between the 2 phases for studying the equilibrium in one phase34,35). With isoxtraction, the crystal surface effect was avoided by dispersing UCB in the organic solution before partition, and also, significant degradation of UCB was avoided. This was confirmed by T.L.C. of the equilibrium phases, which revealed less than 1% oxydation of UCB, exclusively at pH values above 9.0.
The stability of UCB was also demonstrated by the fact that addition of 5 mM EDTA as a stabilizer did not influence the result. With isoextraction, the partition equilibrium could also be attained rapidly. We found that equilibrium could be attained around 48 hours (Fig. 2) and UCB in the aqueous phase was stable after 48 hours. The pH values at 72 hours had shifted somewhat, but It was due to the diffusion of less polasion species through the CHCl3 phase, not to the degradation of UCB.
In this study, we considered the effect of pH, ionic strength, the type and concentration of bile salt, and the presence or absence of micelles. Initially, studies were done with pH ranges from 3. 0–11.0 but later, we found that relative error could occur at lower concentrations. There was no measurable color in the aqueous phase below pH 4.5, which probably reflects the range at which UCB is fully protonated in the diacid form.
Above pH 10.0, significant error could also occur, there were red-brown discolorations in the aqueous phase, which probably reflect the change in conformation and absorbance in UCB due to ionization of CONH groups in one of the end pyrrole rings. So studies were limited from pH 5.0 to 9.5.
As in other studies, we found that increasing pH values enhanced the solubility of UCB in buffered aqueous solutions, indicating that the salts of the monoanion (BH−) and the dianion (B=) of UCB were more soluble than the diacid (BH2) (Fig. 3). UCB dissolves as a diacid and then ionizes in the aqueous phase according to the pH. In buffered aqueous solutions without bile salt (Fig. 4), solubility of UCB was similar to that from dissolution of UCB crystals but it was higher than the values reported by Broderson13) and those by Moroi3). The differences between our study and their prior studies were probably because their incubations were completed in only a few hours, and thus did not approach the equilibrium.
The solubility functions of UCB need to be understood in terms of the molecular interactions among the 3 species of bilirubin (BH2, BH−, and B=) and the bile salt monomers, dinners and micelles. As shown in Fig. 5, increasing bile salt concentrations augmented UCB solubility at all pH ranges, suggesting that all 3 forms of UCB were capable of interaction with bile salts. This result is corroborated by changes in the absorption spectrum31,36) and decreased susceptibility to enzymatic peroxidation37) of UCB in the presence of bile salts. TC below 5 mM (0 and 4 mM) are below the critical micellar values, and 20 and 50 mM are above the critical micellar values.
Isoextraction studies with non-micellar bile salt, TDHC were also performed to compare with micellar bile salt, TC (Fig. 6). There were strong similarities between the TC and TDHC at each concentration. At 4 mM, both bile salts are in the monomer forms, similarity was expected and the results were same at all pH values. At 20 mM where TC is in the micellar form and TDHC is in the monomer form, the results were also similar. The solubilization by TDHC was somewhat lower than TC but not much. At 50 mm, where TC is in micellar form and TDHC is in dimer form, the solubilization was a little better for TDHC than TC at acid pH (pH<7.0), whereas it’s a little better for TC at alkaline pH (pH>7.0). Therfore, BH− may interact hydrophobically with one or more TC or TDHC monomers to produce mixed aggregates, and bile salt monomers, dimers and micelles are equally effective in solubilizing bilirubin.
The effect of biliruin purity was important on partition, and we noticed the fact that different lots of bilirubin yielded different results and even the commercial unconjugated pigment preparations were contaminated with variable amounts of bilirubin III α and XIII α. So we purified the bilirubin by Mc. Donagh’s method21) with a boiling CHCl3 mixture, washing with 0.1 M NaHCO3 and distillation. Tests for purity, discussed by Mc Donagh38) include the following; a) the pigment must be completely soluble in CHCl3; b) treatment with dilute solutions in CHCl3 with 0.1 M NaHCO3 should not extract any color; c) TLC on silica gel and on polyamide should yield a single yellow spot. As shown in Fig. 7, the impurities in the bilirubin increased the partition into aqueous phase, esp. at pH values ≤8.0. The shape of the curve and apparent pK values were also altered.
A number of investigators17–19) have attempted to determine the dissociation constant of bilirubin. However, they have not been able to agree as to the ionization constants of the 2 carboxyl groups. They don’t even agree whether the 2 carboxyl groups have identical or different pK values12,19,39,40).
We have an assumption that BH2 is the only species that partitions between the CHCl3 and organic phases and then ionizes in the aqueous phase according to the pH in micellar and bulk components of that phase.
Then, our study follows this simple model:
Thus, S (Solubility:total UCB) = BH2 + BH− + B= + (B)1, where BH− = (BH2)·K1/H+, B= = (BH2)·K1·K2/(H+)2, and assuming that there is no aggregation of UCB in the CHCl3 phase. Then, S = BH2 + BH− + B= = (BH2) (1 + K1/H+ + K1·K2/(H+)2) = (BH2) (1+10pH–pK1+10pH–pK1·10pH–pK2), and if the BH2 is fixed and pH is known, we can calculate the pK.
Table 1 shows the pK values yielded by computer analysis of the curves from Fig. 8. A chemist would expect a pK of about 4.0, as for other carboxylic acid, but our results are 2.5–3.0 units higher than expected and it’s probably because the intramolecular hydrogen bonds stabilized the acid and then shifted the pK to higher values. The second ionization constant was not apparent at pH 6.0–8.0 which is physiologic for bile likely due to internal hydrogen bonding in bilirubin and the apparent pK2 was 9.2 in 50 mM TC. Our pK values differed from other reporters. Broderson13,40) reported the simultaneous ionization of the 2 carboxyl groups of UCB and Moroi3) reported that the 2 pK values were 6.0 and 7.6 respectively. These different results may be due to their failure to approach the equilibrium. Using these pK values, we calculated the proportions of BH2, BH−, and B= in 50mM TC solutions. The BH− constituted over 90 % of UCB at pH 6.0–8.0, and B= became dominant above pH 9.0. Therefore, it the solubilization of UCB is an appropriate model for bile, BH− accounted for over 90% of the ionized UCB at physiological pH values.
Our results suggest that: a) isoextraction of UCB from CHCl3 avoids the crystal surface effects and follows the model: