A Study on Plasma Prostaglandin E2 Levels in Hepatitis B Carriers and Patients with Chronic Active Hepatitis
Article information
Abstract
Prostaglandin E2 (PGE2), one of the major prostaglandins synthesized in human monocyte and macrophage, is able to modulate T lymphocyte reactivity, such as lymphokine secretion and cytotoxicity. Some immunologic abnormalities such as alteration in the synthesis of PGE2 by monocyte and macrophage or in the response of T lymphocytes to PGE2 can be found in clinical disease.
We measured the plasma PGE2 level in the control group and patients with chronic liver disease.
The results were obtained as follows.
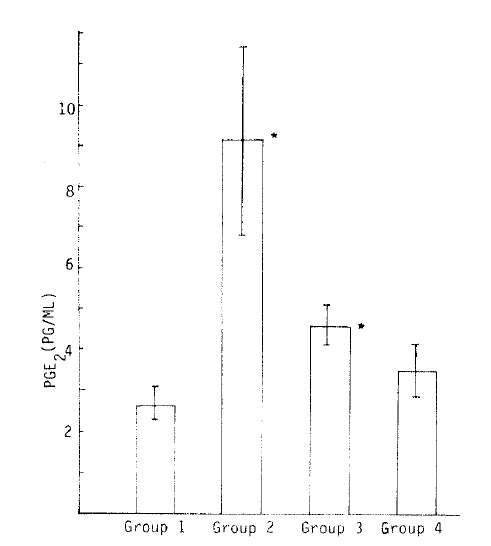
The mean plasma PGE2 level was 2.65 ± 0.69 pg/ml in the control group.
The mean plasma PGE2 level was 9.07 ± 5.89 pg/ml in 15 patients with chronic active hepatitis and was significantly higher than that of the control group (p<0.01).
The plasma mean PGE2 level was 4.65 ± 1.59 pg/ml in 8 patients in the healing stage or stable stage of chronic hepatitis and was tend to decrease. However, this decrease is significantly different from that of the control group.
The plasma PGE2 level was 3.5 ± 0.92 pg/ml in 4 hepatitis B carriers and was not significantly different from that of the control group (p<0.05).
This results suggest that plasma PGE2 can be used for the measurement of cell-mediated immunity and follow-up study in patients with chronic active hepatitis and hepatitis B carriers.
INTRODUCTION
In the pathogenesis of chronic active hepatitis the importance of spell out then abbreviate CMI has been emphasized.
The hepatitis B virus is cleared by cytotoxic T-lymphocyte if the clearing is defected, inflammation progresses1,2).
Goldyne3) and Goldyne and Stobb4) reported that monocytes play an important role in the regulation of several immunologic responses. Monocytes exert this regulatory function which seems to be due to the production of PGE2 is known to suppress various lymphocyte functions (T-cell cytotoxicity, lymphokine production and cytotoxicity). Lymphocyte hyporesponsiveness in cirrhosis has been shown to be mediated by prostaglandins produced by monocytes5), as T-cell proliferative response has been depressed in Hodgkin’s disease6) and PGE2 also plays an important role in the regulation of natural killer cells7,8).
The effect of prostaglandins on the cell is thought to be mediated by their ability to bind to membrane receptors causing an increase in the intracellular cyclic adenosine 5′-monophosphate (cAMP) levels9–12). The authors studied plasma PGE2 assay to determine relationship between the plasma PGE2 and chronic active hepatitis.
SUBJECTS
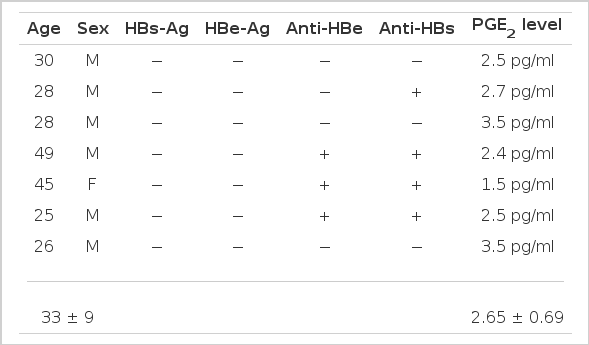
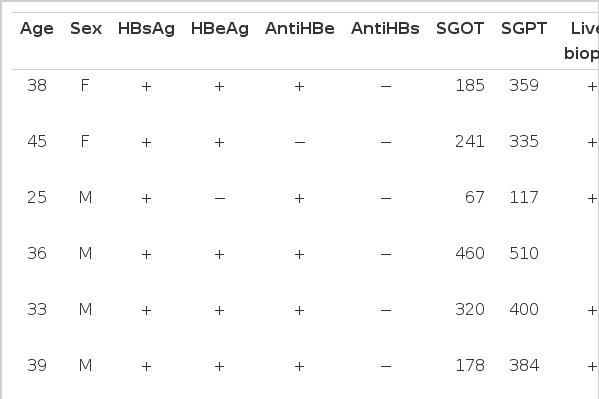
The study group consisted of the patients with chronic active hepatitis and hepatitis B virus carriers who had been hospitalized or visited the Internal Medicine Department, Chonbuk National University Hospital from Jun. 1986 to Aug. 1986. The patients were divided into 4 groups (Table 1, Fig. 1). Group I consisted of 7 normal control subjects who had not been previously exposured to the hepatitis B virus or who were asymptomatic but exposed to hepatitis B virus previously. Gorup II consisted of 15 patients who had been diagnosed as having chronic active hepatitis by liver biopsy and strongly suggested or had chronic active hepatitis by clinical manifestation. Group III consisted of 8 patients who were in the recuperative stage or stable stage of chronic hepatitis. It is based on asymptomatic mild transaminase elevation (lower than 150 U/L and one-third of the previous level) which persist for 3 months and as HBeAg disappeared, AntiHBe appeared. Group IV consisted of 4 patients who were asymptomatic carriers.
METHODS
PGE2 was measured by modified Jaffe’s Method13).
Blood specimens were centrifuged within one hour after collection to separate the plasma. A one milliliter plasma sample was obtained. The plasma was extracted with 3.0 ml of petroleum ether to remove neutral lipids. The aqueous layer was then exposed to 3.0 ml of 3:3:1 ethyl acetate: isopropanol: 0.1 MHcl, apparent pH 5.8 and vortexed, and a mixture of 2.0 ml of ethyl acetate and 3.0 ml of water was added. After further mixing, two phases were separated by centrifugation (2000 rpm for 5 min at ambient temperatures). 3 ml of the 3.5 ml organic phase were removed by aspiration and dried in air at 55°C.
After drying 0.5 ml assay buffer (0.9 Nacl, 0.01 M EDTA, 0.3% bovine L-globulin, 0.005% Tritonx-100, 0.05% sodium azide in 50 mM phosphate buffer pH 6.8) was added and the two phases were separated by centrifugation, 0.1 ml was removed by aspiration. Radioimmunoassay were performed by means of antibody I125 labelled PGE2 (NEN).
RESULTS
The mean plasma PGE2 level was 2.65±0.69 pg/ml in the control group (Table 2).
The mean plasma PGE2 level was 9.07±0.89 pg/ml in 15 patients with chronic active hepatitis and was significantly higher than that of the control group (p<0.01) (Table 3).
The mean plasma PGE2 level was 4.65 ± 1.59 pg/ml in 8 patients in the recuperative stage or stable stage of chronic hepatitis and tended to decrease. However, this decrease was significantly different from the control group (Table 4).
The mean plasma PGE, level was 3.5±0.92 pg/ml in 4 hepatitis B carriers and was not significantly different from that of the control group (p>0.05) (Table 5).

Plasma PGE2 Levels in Patients in the Recuperative Stage or Stable Stage of Chronic Hepatitis (Group III)
The above results suggest that plasma PGE2 can be used for the measurement of cell-mediated immunity and follow-up study in patients with chronic active hepatitis and hepatitis B carriers.
DISCUSSION
Chronic hepatitis is defined as a chronic inflammatory reaction in the liver as shown by liver function tests and histologic studies and that continues without improvement for at least 6 months14).
A group of European histopathologists and clinicians in Zurich in 1968 separated chronic hepatitis into chronic active hepatitis and chronic persistent hepatitis.
Chronic active hepatitis is marked by a chronic inflammatory infiltration involving portal zones and extending into the parenchyme with piecemeal necrosis.
Chronic active hepatitis progress to liver cirrhosis and hepatoma.
The pathogenesis of chronic active hepatitis is till unknown, but the patient having chronic hepatitis B would be expected to have some deficiency of cell mediated immunity1,2).
It also applies to the patients suffering from diseases that depress immunity such as renal failure, malignant disease, and especially those receiving corticosteroids or cancer chemotherapy16). Hepatitis B virus is cleared by cytotoxic T-lymphocyte from infected liver cell, but in patients with a depressed cell activity, the virus is not cleared and can develope into chronic hepatitis.
Thereafter, a plasma PGE2 assay in patients with chronic active hepatitis and hepatitis B carriers performed for the evaluation of the origin of deficiency of CMI.
In 1980 Goldyne3), Goldyne, Stobb4), Galanard17) reported that PGE2 from macrophages ragulated the T-cell activity. Plasma PGE2 is secreted from plasma monocyte and macrophage. Monocytes have a receptor to prostaglandin, so PGE2 suppress the lymphocyte function & natural killer cell activity.
Fisher reported that the pathogenesis of the depressed T-cell proliferative response in Hodgkin’s disease is monocyte-mediated suppression via prostaglandins and hydrogen peroxide18).
Other evidence of CMI suppression due to PGE2 such as reduced monocyte function is found in cirrhosis but in the presence of indomethacine there is a significantly increased the lymphocyte response to PHA-P in patients with cirrhosis5). Lymphocyte hyporesponsiveness in cirrhosis has been shown to be mediated by prostaglandins produced by monocytes.
Patients with malignancy or chronic disease also showed the increased plasma PGE2 levels19).
A substantial evidence of prostaglandins as potent regulator of immune response is accumulating. Inhibition of human T-cell proliferation by PGE2 is well established20–24).
The mechanism of immunoregulation of prostaglandins is that the human leukocyte posses specific and separate receptors for endogenous hormones including β-adrenergics, histamine prostaglandins9,10). This receptor has a high affinity for PGE2 which increases the cAMP levels which cause inhibition of release of lymphokine, lymphotoxin, interleukin-2 (IL-2) for the T-cell proliferation9–12).
Thereafter immunoregulatory action was performed by a decreased amount IL-2 and lymphokine due to increased plasma PGE2 levels25,26).
Prostaglandin E2 also can affect the spleen, lymph node, and bone marrow. The proliferation of cells from spleen, lymph node, bone marrow may be inhibited by prostaglandin E2. The bone marrow and thymus cell were more sensitive to prostaglandin suppression. The sensitivity of various cell populations, the quantities of prostaglandins and augmentation by indomethacin suggested that prostaglandin may modulate cell function in the bone marrow and thymus27).
Natural killer cell activity was also inhibited by prostaglandins7,8). Natural killer cells are a subpopulation of lymphocytes that are cytotoxic to a variety of malignant and normal cells. There especially active in the immunosurveillence role against neoplastic cell. Droller reported that spontaneous cytotoxicity of human peripheral blood lymphocyte against tumor cells can be inhibited28).
In patients associated with depressed natural killer activity, increased production of prostaglandin has been found. So prostaglandins suppress immune reactivities and may be an important set of regulators of natural killer cell activity.
Because the above described mechanisms of immunoregulatory action of prostaglandin E2, the increased PGE2 level which suppress the T4+, T8+ cell proliferation, cytotoxicity was decreased. Under this state, infected hepatitis B virus can not be cleared by cytotoxic T-lymphocyte and progressed into chronic active hepatitis.
We tried the plasma PGE2 level in patients with chronic active hepatitis and hepatitis B carrier because these disease seems to have defect of CMI.
The results were that the plasma PGE2 level in patients with chronic active hepatitis is significantly higher than that of the control group. This fact suggest that plasma PGE2 level has a close reversed relationship with CMI. Because we don’t have a specific methods to predict the prognosis of chronic active hepatitis, plasma PGE2 level will be helpful in evaluating the prognosis of hepatitis patient.